Careful Curation of Care Content: A Case Study of a Technology-Supported Atrial Fibrillation Outpatient Clinic
Renee Noortman 1,2,*, Anne Wil Burghoorn 2, Peter Lovei 1,2, Jos-marien Jansen 2, Eva Deckers 3, Jasper Vermeer 3, Tineke Vinck-de Greef 3, Lukas Dekker 3, and Mathias Funk 1,4
1 Eindhoven University of Technology, Eindhoven, the Netherlands
2 Philips Experience Design, Eindhoven, the Netherlands
3 Catharina Hospital, Eindhoven, the Netherlands
4 Eindhoven Artificial Intelligence Systems Institute, Eindhoven, the Netherlands
Medical care received outside the doctor’s office and the hospital is gaining traction. Lifestyle programs for transmural and remote care are increasingly facilitated by hospitals as part of rehabilitation, by general practitioners as preventative measures, and by various private (health) organizations through consumer apps. What is often overlooked is the time and energy spent on creating the content (i.e., health information and education) in these programs so that it is effective, appealing, relatable, and personalized. In this article, we discuss the elaborate content creation process of lifestyle content for an outpatient clinic for atrial fibrillation patients. We describe the close collaboration between clinicians, design researchers, data designers, and a copywriter, and reflect on how to streamline and formalize the clinical content creation process. Additionally, we highlight opportunities for further content personalization by making it dynamic, more versatile in terms of delivery and expanding the system further into the home context.
Keywords – Clinical Design, Co-Design, Content Design, Data-Enabled Design, Lifestyle Interventions.
Relevance to Design Practice – This paper presents the process of creating quality content for novel patient engagement in the clinical domain and a case study of applying a content co-design approach.
Citation: Noortman, R. R., Burghoorn, A. W., Lovei, P., Jansen, J. M., Deckers, E., Vermeer, J., Vinck-de Greef, T., Dekker, L., & Funk, M. (2023). Careful curation of care content: A case study of a technology-supported atrial fibrillation outpatient clinic. International Journal of Design, 17(3), 79-94. https://doi.org/10.57698/v17i3.05
Received August 23, 2022; Accepted November 14, 2023; Published December 31, 2023.
Copyright: © 2023 Noortman, Burghoorn, Lovei, Jansen, Deckers, Vermeer, Vinck-de Greef, Dekker, & Funk. Copyright for this article is retained by the authors, with first publication rights granted to the International Journal of Design. All journal content is open-accessed and allowed to be shared and adapted in accordance with the Creative Commons Attribution 4.0 International (CC BY 4.0) License.
*Corresponding Author: r.r.noortman@tue.nl
Renee Noortman is a doctoral candidate at Industrial Design, Eindhoven University of Technology (TU/e). She is interested in the role of storytelling and futuring practices in (data-enabled) design processes. In her doctoral research, she combines her love for literature and fiction with design and actively questions the status quo in existing data practices through storytelling and making. She focuses on interactive storytelling in the clinical context to encourage vision-making about the future of healthcare in an active collaboration with Philips Experience Design and Catharina Hospital. She holds a BSc and MSc in Industrial Design from TU/e and has collaborated with University College London Interaction Centre (UCLIC) as a visiting researcher.
Anne Wil Burghoorn is a product owner at Philips Experience Design on care pathways and workflows. As a product owner and design researcher, she focuses on designing solutions that foster meaningful experiences for patients and clinical staff. She works on healthcare transformation by co-creating new healthcare realities with multidisciplinary teams. She holds a BSc and MSc degree in Industrial Design from TU/e.
Peter Lovei is a doctoral candidate at Philips Experience Design and at TU/e in the department of Industrial Design. He has a background in Computer Science. He combines programming and data analysis with a design mindset. He works on scaling up and expanding data-enabled design for the clinical context, focusing on developing micro intelligences for designers, patients and clinical professionals. He is a co-lecturer of the Data-enabled Design master elective course at TU/e.
Jos-marien Jansen is associate design director at Philips Experience Design. She specialises in design research for healthcare innovations and data-enabled design. Her work combines intelligent ecosystems, AI and deep user insights into user needs. She works in multidisciplinary teams with designers, statisticians, data scientists, psychologists, clinicians and patients. Before working at Philips, she worked as a communication consultant and was a producer in the media industry.
Eva Deckers is an innovator in digital healthcare at the Catharina Hospital in Eindhoven. Together with healthcare professionals and a multidisciplinary program team she develops and implements hybrid care solutions. She collaborates with healthcare professionals to develop innovative AI solutions, overseeing a team of technologists dedicated to building a local AI platform and enhancing patient care through algorithm development. Before her hospital role, Eva was design director at Philips Experience Design, pioneering data-enabled design, shaping customer-driven strategies and driving the SaaS and Data and AI transformation. With a PhD from the department of Industrial Design at TU/e, Eva’s career has been marked by a strong connection to research activities.
Jasper Vermeer is a resident cardiology in the Catharina Hospital of Eindhoven, where he is also currently pursuing a Ph.D. He has studied medicine in Maastricht, where his fascination for the heart started. He has always been interested in improving the treatment outcomes for patients. At this moment, he is conducting research on the management of heart rhythm disturbances such as atrial fibrillation. The focus of his Ph.D. thesis will be on the impact of lifestyle choices and modifiable risk factors on heart health and treatment outcomes. Furthermore, together with the Eindhoven University of Technology he is currently working on AI models to predict the success of invasive procedures for rhythm disturbances, ultimately with the aim of improving the management of patients with atrial fibrillation.
Tineke Vinck-de Greef is a nurse practitioner in the cardiovascular department at Catharina Hospital in Eindhoven. She guides and treats patients with chronic heart failure in a multidisciplinary setting at the heart failure outpatient clinic and treats patients on their risk factors for atrial fibrillation on a lifestyle intervention trial. She is also active at the Netherlands Heart Network (NHN) and a member of the science committee at the Catharina Hospital. As a nurse practitioner, she wants to make the connection between medical treatment and patient-centered nursing.
Lukas Dekker is a cardiologist-electrophysiologist in the Catharina Hospital in Eindhove. His clinical and scientific work focuses on preventing and treating atrial fibrillation and ventricular arrhythmias. Dekker is a professor at the department of Electrical Engineering and Biomedical Engineering at TU/e. An overarching theme in his work is value-based healthcare (VBHC). Dekker is co-founder and board member of the Eindhoven MedTech Innovation Center (e/MTIC) and of the Netherlands Heart Network (NHN).
Mathias Funk is an associate professor in the Future Everyday group in the Department of Industrial Design at TU/e. He has a background in Computer Science and a Ph.D. in Electrical Engineering (from TU/e). His research interests include methods and tools for designing with data, designing systems of smart things, and interfaces for musical expression. In the past, he has researched at ATR (Japan), RWTH Aachen, Philips Consumer Lifestyle and Philips Experience Design, Intel Labs (Santa Clara), National Taiwan University of Science and Technology, and Natrional Taiwan University. He is also the co-founder of UXsuite, a high-tech spin-off from TU/e.
Introduction
A significant share of the most prevalent diseases in the developed world nowadays can be linked to lifestyle concerns such as nutrition, lack of physical activity, and smoking (Forastiere et al., 2016). Patient education and motivation are key concerns in lifestyle medicine, and research has shown that patients who are engaged in their own healthcare make less long-term care costs (Kuwabara et al., 2020). However, there is a gap between public health recommendations and their implementation in patients’ lives (Collings et al., 2022). A wide array of clinical and healthcare content1 exists, ranging from validated websites (e.g., World Health Organization, 2010) to hospital leaflets, to short videos on TikTok and Instagram (Bruno, 2020; Manfredini, 2021).
In times of digitalization and the recent COVID-19 global health crisis, healthcare information consumption has changed, and will continue to change (Kuwabara et al., 2020; Soroya et al., 2021). When large amounts of information were rapidly released during the pandemic, care consumers experienced greater difficulty in assessing the quality of health information than before, as official health sources were presented next to popular social media articles (Soroya et al., 2021). Validated healthcare content must increasingly compete with easily accessible and highly engaging content available through social media and consumer products that can hold incorrect or even harmful information due to lack of regulation (Madathil et al., 2014). Due to the growing number of resources and easier access to health information online, patients can experience information overload, and it is important that clinicians can help their patients gain access to reliable and understandable content (Chatterjee et al., 2021; Soroya et al., 2021). Digital health technologies have been shown to offer opportunities to communicate healthcare information to patients over time, although these technologies are also restricted in their effects due to low usage rates (Chatterjee et al., 2021) and can be problematic in stimulating a digital divide (Yoon et al., 2020). While providing lifestyle information to patients is important, merely informing them about clinical facts is not enough to stimulate change (Kelly & Barker, 2016). To cater to better patient engagement and experience, health organizations seek to personalize patient experiences and embed sensing technology for more accurate treatment and diagnosis (Dinh-Le et al., 2019).
In this quest for more predictive, precise, preventive, and personalized healthcare (Flores et al., 2013), healthcare designers are looking for ways to bridge gaps between the various organizations that make up our healthcare system and to achieve a meaningful impact on patients’ everyday contexts. As a result, challenges in the healthcare domain are becoming more complex, holistic, and multidisciplinary (Sanz et al., 2021). This can be seen in an increase in the application of co-creation, co-design, participatory design and data-enabled design processes applied to healthcare problems and emerging care structures (Smith & Ashby, 2020; van Kollenburg & Bogers, 2019). The increasing complexity of multi-stakeholder processes also necessitates that design practice assumes a bigger role in healthcare innovation and that clinicians engaging in medical innovation processes as domain experts are developing their design skills in this process as well. Through their experience with designing intuitive and engaging experiences, experience designers could provide valuable input in the ongoing structural changes in healthcare (Niedderer et al., 2017). The process of meaningful clinical content creation is complex and deserves a more prominent role within design practice (Mahmud et al., 2013). Currently, there are good examples of work that focused on content creation and validation (Groeneveld et al., 2019; Singleton et al., 2021) and content delivery (Seo et al., 2021; St. Amant, 2015), but little work has explored the (clinical) content design process in detail (Kramer et al., 2020). In this article, we aim to shed light on the elaborate process of creating high-quality clinical content that engages patients in their own care path. We tackle this by providing a list of requirements for the content and explaining how we tackled the process with our multidisciplinary team.
For this research, we specifically look at the care pathway for patients with atrial fibrillation (AF in the following). AF is the most common cardiac arrhythmia, and its prevalence is expected to rise due to an aging population with an unhealthy lifestyle (Kornej et al., 2020). Patients with AF have a higher risk of hospitalizations for stroke and heart failure and have a lower quality of life (Cherian et al., 2017). Lifestyle changes are essential for managing atrial fibrillation (Chung et al., 2020). Several lifestyle risk factors, such as obesity, hypertension, and alcohol use, have been linked to AF (Chung et al., 2020). Treatment of symptomatic AF consists of medication and catheter ablation. During the ablation procedure, a catheter is inserted in the patient’s femoral veins and guided to the heart while the patient is sedated. The cardiologist then uses the catheter to induce electrical energy that leads to the development of scar tissue in the heart that prevents the onset of atrial fibrillation (Mayo Clinic, 2022). However, long-term treatment outcomes are poor, and patients might experience recurrences of atrial fibrillation. Managing lifestyle risk factors may improve ablation outcomes (Pathak et al., 2015), and education is an essential component of care for these patients (Salmasi et al., 2019).
In this case study, we demonstrate how a co-design process between experience designers and clinicians helped to combine clinical content with a patient engagement platform aimed at personalized and value-based healthcare. Within this collaboration, we sought to create content that is appropriate for the patient population in terms of information, user experience and language. This case study is part of the POP trial (Vermeer et al., 2023), which concerns the redesign of an outpatient clinic for AF patients, introducing a lifestyle change program for patients with atrial fibrillation, prior to undergoing a catheter ablation. By providing lifestyle support early on, the aim of the clinic is to alleviate symptoms and increase the chances of a successful ablation. In this paper, we present the collaboration process with the clinicians as a case study of how to systematically create, validate, and deploy healthcare content. We provide details on our methods for (1) care pathway redesign, (2) content strategy, (3) content creation, (4) content validation, (5) implementation, and (6) content delivery and share our experiences and challenges in each of these steps. We reflect on our content design process, including team composition, design capabilities, clinical capabilities, and the content creation process. Finally, we generalize our findings towards recommendations for others pursuing healthcare content design processes. We close with suggestions for further research in healthcare content development and delivery.
Background
To design the content for the POP trial, a rich body of work on lifestyle coaching and behavior change strategies was consulted. More specifically, existing approaches to supporting lifestyle change as part of the atrial fibrillation care pathway were reviewed.
Lifestyle Coaching
There is overwhelming clinical evidence that lasting lifestyle improvements are beneficial for both life expectancy and quality of life (Li et al., 2018; Wolin et al., 2007). Designing for sustainable lifestyle change requires the integration of design and behavioral science practices (Cash et al., 2022). Lifestyle improvements require long-term behavior change, which is more successful when using a personal approach and analysis of each behavior and influencing both social and environmental conditions (Khan & Lee, 2013). Furthermore, previous research has shown that AF patients also have a need for emotional appraisal and anxiety education as part of their treatment (Salmasi et al., 2019). Lifestyle coaching apps have applied behavior change techniques such as offering rewards, providing users with feedback, and encouraging them to compete with other users (Villalobos-Zúñiga & Cherubini, 2020). The use of digital technology can make information more easily accessible to users and simultaneously also lower the burden on clinicians (Sanz et al., 2021). Designers in the field of behavior change find themselves at the junction between designing solutions that are intuitive to use but simultaneously promote positive societal change (Niedderer et al., 2017). In this work, we have incorporated goal setting in the content and have focused primarily on integrating actionable advice regarding incorporating healthy behavior into everyday life.
Content Design
Effective solutions to promote a healthy lifestyle rely heavily on content: information and notifications that are sent out to motivate individuals to change their behavior in a particular way. Healthcare content can be shared with patients in different formats, including brochures, web interfaces, mobile apps (Villalobos-Zúñiga & Cherubini, 2020) and text messages (Chow et al., 2015). A previous trial has shown that cardiovascular patients who consistently received text messages with lifestyle advice for six months experienced a moderate improvement in several clinical measures (i.e., blood pressure, smoking, and BMI) compared to similar patients who did not receive these texts (Chow et al., 2015). Aside from information about their condition and the procedure, AF patients and clinicians have also indicated a need for emotional appraisal and content that discusses clinical information in an applied context, such as how AF will affect their quality of life and daily activities (Salmasi et al., 2019; McCabe, 2011). The possibility to interact with professionals in real-time is also appreciated by patients (Salmasi et al., 2019).
When creating new healthcare content, rather than reusing content from other care pathways, the content can be tailored for the specific context of use, meaning that patients receive accurate and meaningful information (Aldridge, 2004). However, the content creation process can also be challenging because of a diverse patient population with varying literacy and information needs. Thus, it is important that the language level of the content is as simple as possible (Aldridge, 2004). Prior work has also discussed how to streamline and tailor the content creation processes. Methods include generating a widely applicable library of universal content to be personalized by local healthcare professionals (HCPs) (Molapo & Marsden, 2013). Previous work has also highlighted the importance of collecting evaluation data when designing health communication programs (Kreps, 2014).
Co-creation and Co-Design
Co-creation and co-design are well-established design processes that focus on the involvement of stakeholders as designers in the design process (Sanders & Stappers, 2008). Co-creation refers to acts of collective creativity, whereas co-design specifically refers to collective creativity with a group of both designers and other communities of practice to create new, desired futures (Holmlid et al., 2015; Sanders & Stappers, 2008). A review of co-design processes by Sanz et al. (2021) has demonstrated that the co-design of digital healthcare solutions increases the chances for success in practice. Previous co-design work in the healthcare domain has applied diverse methods to involve clinicians in the design process: interviews, meetings, presentations, workshops, surveys, and user testing sessions (Sanz et al., 2021). In the health domain, the high workload of clinicians can complicate their involvement in co-design work (Sanz et al., 2021). Research co-design has also been applied to set up shared studies between designers and clinicians, though these practices have thus far not been widely documented (Slattery et al., 2020).
Case study: Content Creation for a New AF Outpatient Clinic
In this case study, we describe the content creation process for a new nurse-led AF outpatient clinic that is focused on lifestyle. The clinic provides treatment for multiple risk factors, targeting lifestyle behaviors associated with increased AF symptoms through content programs. The clinic is currently being validated in the POP trial, a randomized clinical trial designed to investigate the benefits of nurse-led integrated lifestyle care in patients referred for AF ablation. The ClinicalTrials.gov Identifier is NCT05148338. A total of 150 patients will be enrolled and randomized in a 1:1 ratio to the intervention group and control group. The patients in the intervention group receive treatment and coaching on risk factors associated with their lifestyle prior to their catheter ablation. At the time of publishing this paper, this clinical trial is still onboarding patients.
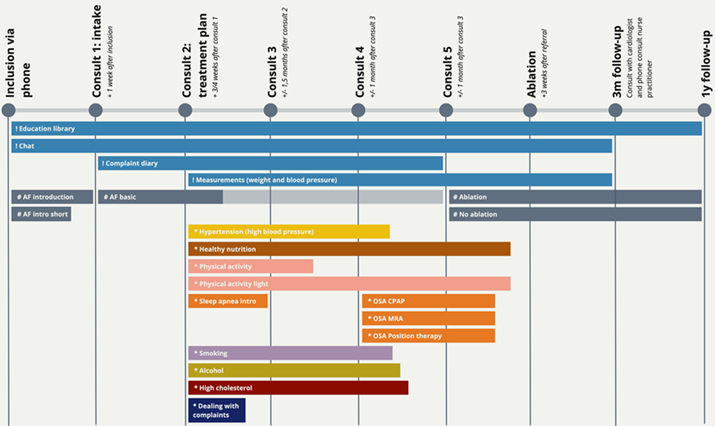
The novelty of the program compared to the existing care pathway is that it is nurse-led and that the lifestyle content is deeply embedded in the clinical pathway through the involvement of the nurse practitioner. This means that patients receive information about the procedures and face-to-face that they have in the hospital, and that all information is personalized for patients in this specific care trajectory for AF. For an overview of care pathway of the clinic, see Figure 1. The difference between this clinic and the care previously provided at the hospital consists of introducing the engagement platform and additional consults. In the traditional care pathway, patients would only see the care team for an intake, an information session on the ablation procedure and the ablation itself. The lifestyle program in the intervention group will delay the ablation procedure for up to six months. Still, the lifestyle change will likely already alleviate patients’ symptoms after the intervention and increase the chances of success for the ablation. Patients who fit the inclusion criteria (i.e., they are eligible for the ablation procedure and would benefit from lifestyle treatment) are informed about the study procedures before signing up for the trial and participate voluntarily.
Figure 1. Timeline of the clinic from the patient perspective: illustrating the physical interactions (the circles), the platform functionalities (marked with !), the general AF content that every patient gets (#) and the content programs that are personalized per patient (*). (Click on the figure to see a larger version.)
Content
The purpose of the content created for the POP trial is to provide education on and guidance in lifestyle changes associated with the reduction of AF symptoms. The content supports staff in guiding lifestyle changes for patients. Currently, clinicians typically share information about the ablation procedure and general lifestyle advice with their patients by handing them leaflets. The clinicians can also share these with patients online through the hospital website. However, the information in these leaflets is generic, and there is no way to schedule content to be delivered at the right time other than doing this manually on a per-patient basis. With the trial, we set out to deliver the content gradually and to deliver content to the patients that are relevant to their personal situations.
During the trial, the patients in the intervention group receive personalized lifestyle content through the online Engage platform. The nurse practitioner selects which content patients receive based on the most significant risk factors for their AF symptoms. This content consists of articles of around 50-200 words with attached images, links, and (external) videos (see Figure 2 for an impression of different content items). Articles were combined into collections to comprise personalized lifestyle programs, each targeting the improvement of a specific lifestyle risk factor.
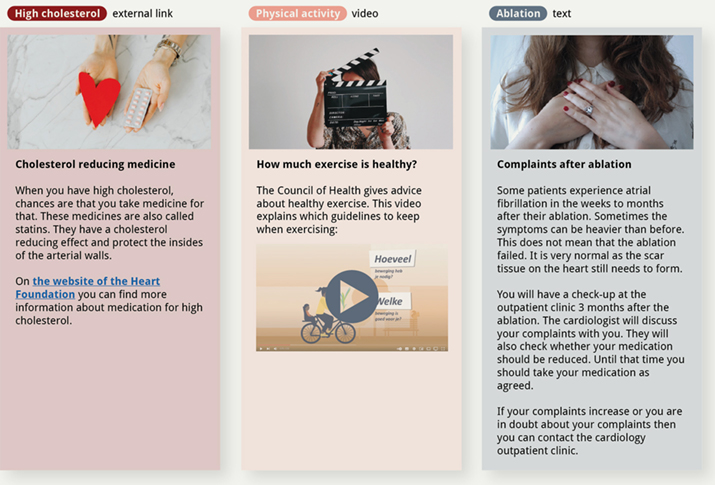
Figure 2. Three examples of articles of different types: external links, video, and text. All external links and videos were verified and approved by the clinicians. The content was translated from Dutch for this overview.
Content Delivery Platform
The targeted solution is a digital patient engagement platform called Philips Engage, see Figure 3, through which patients can access content, set health goals, and chat with their clinicians. Philips Engage is an existing tool that has been used in previous studies; it was adapted to the context through the content. The patient opens the platform in their web browser and can access the content as it is released. The healthcare professionals also have access to a healthcare professional dashboard where they can check whether the patients have engaged with the content and made progress with their personal goals for the risk factors. Patients and their clinicians can also chat through the platform, e.g., to ask for clarification about the content or to ask for more specific advice. In case of urgency, patients are still instructed to call their nurse practitioner or the emergency room.
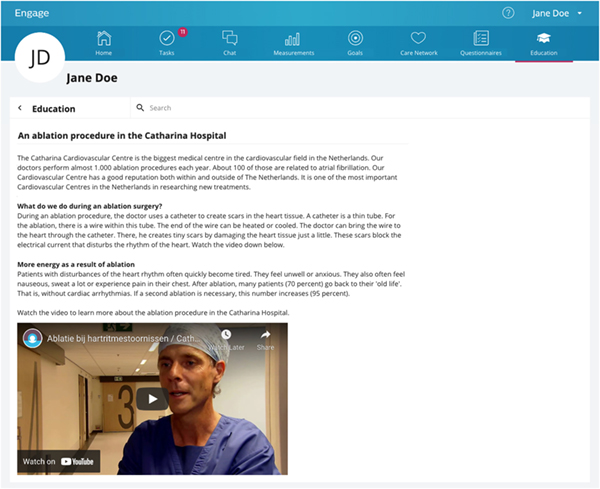
Figure 3. Example content item on the patient engagement platform with general information and a video translated from Dutch.
Methods
In this article, we focus on the content creation process, which was done through an extensive co-design trajectory that lasted approximately one year. Key requirements for the content were set at the beginning of the process based on experience in previous projects (Jansen et al., 2020; Noortman et al., 2022) and literature on clinical content creation (Aldridge, 2004; McCabe, 2011; Salmasi et al., 2019). See Table 1 for an overview of the requirements.
All content for the care programs was created by a core team of designers, a copywriter, and two clinicians: the nurse practitioner who would primarily run the outpatient clinic and the cardiologist in training who is the lead investigator of the clinical trial. See Table 2 for a detailed overview of all stakeholders involved and their responsibilities.
Table 1. Overview of content requirements, requirements labeled for reference.
| Type | The content should… | Source |
| Information | Explain possible causes of AF and highlight the importance of lifestyle changes (I-1) | McCabe, 2011; Salmasi et al., 2019. |
| Explain the consequences of AF and acknowledge anxiety (I-2) | McCabe, 2011; McCabe et al., 2020; Salmasi et al., 2019. | |
| Discuss realistic treatment goals (I-3) | McCabe, 2011. | |
| Provide patients with actionable steps to take towards treatment goals instead of only abstract clinical information (I-4) | McCabe, 2011; Salmasi et al., 2019; Jansen et al., 2020. | |
| Help patients assess perceptions about the effects of AF on their psychosocial well-being and everyday life, and address emotional needs (I-5) | McCabe, 2011; Salmasi et al., 2019; Jansen et al., 2020. | |
| Include patient testimonials or stories (I-6) | Salmasi et al., 2019. | |
| Be clinically validated and reliable (I-7) | McCabe et al., 2020; Noortman et al., 2022. | |
| User experience | Include visuals to illustrate concepts or procedures (U-1) | Aldridge, 2004; McCabe, 2011. |
| Be tailored to what the patient wants to know (U-2) | McCabe, 2011. | |
| Include interactive elements to encourage patients to use the material (U-3) | Aldridge, 2004. | |
| Individualize risk communication based on understanding, interest, resistance to therapy, and educational background (U-4) | Salmasi et al., 2019. | |
| Language | Be written in Dutch at the B1 level (Little, 2006) for those with lower literacy in the Dutch language (L-1) | Aldridge, 2004; Jansen et al., 2020. |
| Use the second-person perspective instead of the first or third person and use active voice (L-2) | Aldridge, 2004. | |
| Use plain, consistent language, short words, and short sentences (L-3) | Aldridge, 2004; McCabe, 2011. |
Table 2. Team composition, number of members in each role between brackets.
| Team | Roles | Responsibilities |
| Content development | Design researchers (2) Service designers (2) |
Defining the structure for content care programs, collecting and writing content items. |
| Copy writing | Copywriter (1) | Reviewing the content items generated by the content development team and rewriting them in B1 level Dutch. |
| Content validation | Nurse practitioner (1) Cardiologist in training (1) Clinical experts (7): dietician (2), psychologist, pulmonologist, OSA nurse, nurse smoking cessation clinic, nurse practitioner physical activity |
Reviewing the various versions of the content items for clinical accuracy and their timing within the care pathway. |
| Data design | Data designer (1) Platform support (3) |
Setting up the data infrastructure to manage patient accounts, streamline content delivery and timing, and assign care programs to patients. |
For almost the entire year, the team met on a weekly basis for an hour. In addition, the clinicians reviewed the content continuously. Sixteen care programs were created in total, with 231 unique content items between them. Five of these care programs are general care programs and are sent to all patients in the intervention group. The other eleven care programs are activated by the nurse practitioner based on relevance for individual patients. We refer to those as risk factor programs. These risk factors are hypertension (high blood pressure), unhealthy nutrition, high cholesterol, sleep apnea (OSA), poor physical activity, excessive alcohol consumption, and smoking. The selection of care programs is personalized for each patient, based on the assessment of the nurse practitioner in the intake consultation meeting. The content on the platform is scheduled to be released to the patients over time and replaces the brochures that would otherwise be handed to them in the first consult.
After this process had ended, the design researchers analyzed the process by creating a detailed timeline. The activities on the timeline were then split into six distinct steps that could be followed by similar teams in the future. In the following sections, we describe in detail how we performed each of the steps in the case study. An overview of the process can be found in Figure 4. In the discussion, we then analyze how the steps contributed to the co-design of content that fits the requirements listed in Table 1.
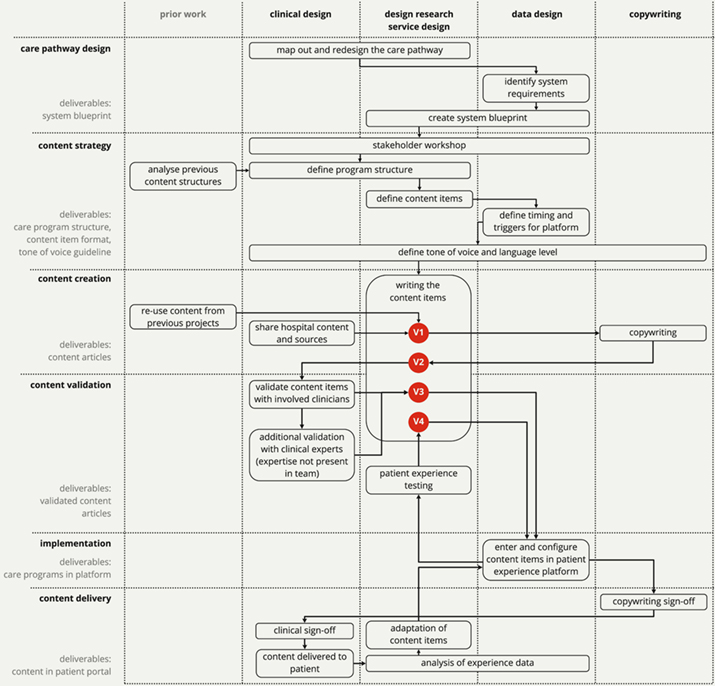
Figure 4. Flow overview of the content creation process.
Care Pathway Design
Duration: two months (part-time) by a multidisciplinary team including two clinicians, one design researcher, one service designer, and one data designer Number of iterations: 5 |
We started the process with getting an understanding of the existing care pathway. Through multiple collaborative with the team, we mapped out the steps that patients currently go through from their initial AF diagnosis to the ablation. From this overview, we designed the care pathway for the new clinic. This care pathway was used as a starting point to define the most appropriate times to support patients in improving their lifestyles. This resulted in a blueprint for the new AF outpatient clinic with risk factor treatment programs that were not part of the AF treatment before. This step took two months in total as the designers had to sensitize towards the context and the existing procedures and systems that were already in place in the hospital.
Content Strategy
Duration: one month (part-time) by a multidisciplinary team including two clinicians, one design researcher, one service designer, and one data designer Number of iterations: 3 |
In the content strategy phase that followed, the structure of the care programs was defined. The content strategy phase was kicked off with a co-design workshop to align clinical, design, and behavior change goals. These were then used to define the strategy for the use of content in the care pathway. The team identified the topic areas that needed content to support them and at what time in the pathway that would be relevant (e.g., explanation about wound care after ablation was not required until the final weeks). The first version of the article titles and the timing of the articles were set up. In alignment with the care program structure and the Engage platform, the content format was defined: patients would be assigned to approximately 2 to 5 care programs, and each of these would receive a content item roughly once every three days. The content items would offer a balance between informative content, tips, and challenges for the practical application of the lifestyle advice. Additionally, the timing of content was aligned with the care path (e.g., monthly face-to-face consultation meetings that the patients would have with the nurse practitioner and the risk factor care paths).
Content Creation
Duration: five months (part-time) by a multidisciplinary team including two clinicians, one design researcher, two service designers, one data designer, and one copywriter Number of iterations: 2 |
Once the care program structure and content format were defined, the team moved to the content creation phase. The content creation phase started with the creation of an outline for the content articles in the programs based on interview data and experience from previous content projects (i.e., Bogers et al., 2018; Jansen et al., 2020; van Kollenburg et al., 2018; Noortman et al., 2022). This outline was discussed with the involved clinicians to further fine-tune the number of articles in the programs and the length of the individual care programs.
From the outline of the care programs, a draft outline for each individual content item was created. Input from the clinical practice (e.g., hospital leaflets, videos), as well as reusable material from previous projects, was used to create the first version of each article. Behavior change strategies regarding goal setting and self-reflection were also considered. The copywriter polished these draft content items further, after which the design research team reviewed them in consultation with the clinicians, checking whether the essence of the original message was captured correctly. In some cases, feedback was sent back to the copywriter, who further adjusted the articles.
Content Validation
Duration: one month (part-time) by a multidisciplinary team including eight clinicians, one design researcher, two service designers, and one data designer Number of iterations: 2 |
In the validation phase, the team involved the clinicians that would be involved in each of the risk factor programs (e.g., a dietician checked the nutrition protocol) to check alignment with the care path that the patients would receive. They checked the medical accuracy of the messages based on their expertise, as well as whether the items were appropriate for the patient population. The experts included dieticians, a nurse practitioner of a cooperating hospital, a psychologist, a nurse from the smoking cessation clinic, a pulmonologist, and a sleep specialist of a cooperating hospital. Most changes during this phase were minor and could be implemented by the design research team directly. In a few cases, the copywriter was consulted again to do a full rewrite of a content item.
The validation process also included patient experience testing, for which one of our design researchers acted as a participant. We set up a staging environment of the patient engagement platform. From an experience perspective, it was important that the test patient received the messages in the same way that a future patient would. This way, we could test whether the content was scheduled correctly and could once more review the content from the patient’s perspective to check for inconsistencies. The data designer collected the necessary updates for implementation.
Implementation
Duration: one and a half months (part-time) by a multidisciplinary team, including one service designer and one data designer Number of iterations: 1 |
After all validation steps were concluded, the implementation began. During this phase, the team finalized the timing of each article in the program to ensure an optimized patient experience if a patient received multiple care programs at the same time. Finally, the programs and articles were made ready for implementation into the software platform. This included linking the right images to the right items and making sure that all settings for the content delivery were set correctly for each care program (e.g., the date the content would become available, whether it was a task or an information item, and when it would expire).
Content Delivery
Duration: one day for the sign-off by a multidisciplinary team, including two clinicians, one design researcher, one service designer, one data designer, and one copywriter. Number of iterations: 0 |
In the final phase, before sending the content to the patients, there was an official sign-off for the entire team, including the nurse practitioner, cardiologist in training, and the copywriter. This final sign-off was important as this was the moment that the clinicians officially approved the content from the perspective of medical accuracy, and the copywriter officially approved the B1 language level and the textual quality of the content. Afterward, once the patients were correctly onboarded in the patient engagement platform, the content items were delivered based on the scheduled format.
Process Reflection
After completing the content creation process, we reflected on the created content according to the requirements set at the beginning of the process. Overall, through the process detailed above, we were able to create content that adheres to the majority of these requirements. In the following, we elaborate on how we ensured that the requirements were met throughout the process and where we still see room for improvement.
Additionally, we describe the challenges we faced throughout the different phases of the process and the insights that we gained in relation to these phases. The core team spent a total of approximately 7.5 person-months on the creation of this content over the course of a year in each of the steps mentioned above. It must be noted that a lot of prior work was available from previous studies (Jansen et al., 2020; Noortman et al., 2022), so the cumulative time investment is higher. We close this section with a preliminary analysis of the patients’ experience with the platform based on a small sample of patients (N = 7) who were interviewed about their experience with the content in the clinical trial.
Information
To ensure that the care programs and the individual content items were clinically appropriate for our patient population and to make use of the vast body of knowledge with regard to AF as a condition (I-1, I-2, and I-7), the involved clinicians reviewed all care programs and content items multiple times. A special program was also developed that discussed anxiety to be activated for the patients who wanted more information on this (I-2). Goal-setting strategies were discussed in each of the treatment programs, and the use of the patient engagement software also made it easy for patients and clinicians to evaluate the goals throughout the treatment program (I-3). The goal setting was also supported by actionable tips (I-4) that were provided to patients to directly make small changes in behavior part of their everyday routine (e.g., by providing healthy recipes). Psychosocial and emotional needs (I-5) were addressed through articles that shared suggestions about how to talk to loved ones about your AF and how to make behaviors last during social gatherings (e.g., reducing alcohol consumption at social events). Additionally, there were articles that contained interviews with other AF patients, and resources were shared to help patients get in touch with peers through patient associations (I-6).
User Experience
To improve the user experience, we made the content attractive for people with different learning strategies by including visuals and videos in the content (U-1). We made use of existing videos that the hospital had previously posted online, as well as visuals that the clinicians already used to explain procedures to their patients. As we did not have an experienced visual content creator on our team, we were limited to reusing materials. Including someone with visualization skills in the multidisciplinary team would have helped us to bring this to the next level. One of the steps we took towards tailoring the content to patient information needs (U-2) was to share content at the moment that it would be most relevant to patients. While, in theory, the content items aligned with the timing of the consults, we quickly learned that, in practice, there would be quite some variation in the timing of these meetings based on clinician and patient availability, as well as external factors such as national holidays. Unfortunately, the interactive elements (U-3) were limited due to the functionalities of the platform we used. However, some interaction was encouraged through the goal-setting module and the everyday activities that were proposed in the content. Personalized risk communication (U-4) was achieved through the assignment of the different programs, though we see opportunities to further personalize within these programs to achieve even better results in the future.
Language
All content within the system was written in B1-level Dutch (Little, 2006) (L-1). The copywriter was essential in achieving this goal and he was experienced in writing content targeting this level of language proficiency. Occasional conflicts did arise when the clinicians expressed that they considered some of the content to be pedantic and that it did not fully acknowledge what the patients would already know. As the tone of voice had to be generalized for all patients, we had to consider that some patients might benefit from plain language while others might prefer more details and scientific information. Oversimplification, on the other hand, could also lead to clinical inaccuracy or statements that came across as too bold. Here, there was a conflict regarding engagement, as we knew from previous studies that a more colloquial tone resonated with many patients. The second-person perspective was applied in most articles (L-2), which was especially important in the articles that were aimed at motivating patients to undertake action. To make sure that the language was consistent (L-3), the clinicians helped assure that the correct terminology was used, and the copywriter made sure that this terminology was consistently used in all articles and that all abbreviations were explained.
Collaboration
While we initially feared that some of the co-design activities might be too time-consuming for the clinicians, we found that they were willing to free up the time as they saw how the time investment would benefit their patients and contribute to better care standards in the long term. Due to the COVID-19 pandemic, much of our collaborative work took place online, which proved fruitful as the clinicians could easily provide their feedback through Miro (see Miro website: https://miro.com/) boards. We worked asynchronously on the content items, which meant that the clinicians could fit it into their day-to-day routine when they had the time. We see our fluent collaboration with the clinicians as a highlight and an important requirement for the successful implementation of our data solutions in practice. By collaboratively designing the care pathway, it was much easier for us to anticipate the situation for the patients and the implementation of our envisioned system in the hospital.
Analysis of Experience Data
We set up the patient engagement platform so that we can analyze how the patients participating in the clinical trial experience the content. The following meta-data is collected through the platform: (1) date and time of login to the system, (2) date and time when patients open content items, (3) date and time when patients mark a content item as read, and (4) date and time for when the content item is scheduled. As the clinical trial is currently ongoing, no conclusions can be drawn from the collected experience data yet. However, we are already looking at preliminary data from the patient engagement platform based on the first 29 participants who completed the study. Throughout the study, these patients opened and read 2578 content items, which amounts to 79,64% of the total items sent out. If we exclude the protocols that were only assigned to less than five patients, the AF basic protocol was read the least at 56,09%, whereas the AF introduction protocol was read the most at 91,92%.
On top of the quantitative use data from the platform, seven patients were interviewed about their experience in the trial (see Table 3 for an overview of interview participants). These patients were interviewed at different moments throughout their care trajectory to get a representative sample of new users of the platform and patients who had been using it for a longer while. The interviews were transcribed and clustered into themes. For this article, we clustered all quotes about the content and the interaction with the content in the platform and summarized these findings below for a first impression of the patient experience.
Table 3. Overview of interview participants.
| Participant | Gender | Age | Timing of interview |
| P1 | M | 60 | After consult 2 |
| P2 | M | 48 | After consult 3 |
| P3 | F | 67 | After consult 4 |
| P4 | M | 72 | After consult 4 |
| P5 | M | 71 | After consult 6 |
| P6 | M | 59 | After referral for ablation |
| P7 | F | 60 | 3 months after ablation |
The interview participants indicated that the platform was clear (P2), structured (P1, P2), and easy to use (P5). For most of them, the content was a repetition of information that they had previously received, but they appreciate having it on the platform so they can reference it in their own time and show it to their partners and family, like P6 indicates: “I read all [the examples about nutrition] in the evening, and discussed with my wife, like ‘look, this is also part of it’.” P4 even indicated that his wife uses the platform more than he does. Through the interviews, we also learned that most patients already know a lot about the lifestyle that is recommended by the health guidelines, especially regarding nutrition: “For example, with the dietician, I already know a lot of that stuff. I have been struggling with my weight for years, so I already know so much about that stuff that it’s not very useful for me to look for more” (P3). However, patients also indicated that while not all the information was new, it was very useful to have it in one place, and to know that the sources are reliable, as opposed to when they would find the information online themselves. Additionally, having everything in one place reassured them that their healthcare professionals in the outpatient clinic were also aware of the other trajectories they were going through, like P6 indicated: “The trajectory that you started with Engage is the red thread and if that didn’t have the information about sleep I would wonder: oh, but do they know that I’m also doing the sleep stuff?”
Unfortunately, the platform is not as accessible as the participants would prefer because they must use a code they receive in their e-mail each time they sign in. This meant that, while we carefully designed the timing of the delivery, they would wait until a couple of content items had accumulated and then tick multiple items off in one go.
The articles were considered short, but the participants also indicated that this was convenient, and P3 added that when she was curious about a topic she would look for more information by herself. Participants indicated that the content by itself did not necessarily feel personal to them, but the overall care pathway with all elements combined (i.e., content, consults, the chat functionality and the measurements in the platform) did feel tailored to their situation. In particular, they greatly appreciated how the platform facilitates a connection between the physical care they receive in the hospital and their home environment. P6 also explains how it saved him travel time: “Well, the combination of physical attention and the attention from the system [works] because without that, I would have needed to come to [the city] for every little thing. This system helps you to take in the information when you are ready for it, and you don’t have to travel [to the hospital]. So it saves a lot of time; I found that combination really valuable.”
Finally, some of the participants had specific requests for extra information, hoping that it could cover more personal experiences of other patients and explain why some patients have had the ablation procedure three or four times before it was successful (P6). Further integration with other care pathways was also suggested. For example, P3 had to get an MRI and had expected to receive information about that in the platform, too.
Recommendations for Content Co-Design
The case study was streamlined based on earlier content creation processes that the team members had been a part of (Bogers et al., 2018; Jansen et al., 2020; van Kollenburg et al., 2018). The process shown in Figure 2 can thus be seen as (1) the product of an accumulation of knowledge of 5+ years and (2) a suggestion for designers who are working on content creation and who are looking for co-creating clinical content with healthcare professionals.
Design and Clinical Capabilities
Besides the fact that our team was composed of both designers and clinicians, the multidisciplinary nature of the team was also expressed in the skill sets of the team members. Table 4 lists the design and clinical capabilities that we consider vital for a clinical content creation process. Over the course of the project, each of us has also gained new knowledge and skills for each other by working so closely together between industry and the hospital.
Table 4. Overview of design and clinical capabilities we consider required for a content creation process.
| Explanation | When | ||
| Design capabilities |
Content strategy | Designing care programs, defining a sequence of content items, and defining how and when these get delivered to patients in an engaging and timely fashion |
|
| Service design | Being able to design the patient journey from start to finish |
|
|
| Design research | Being able to research the patient journey, understand patient needs and use them to inform design. Knowledge of existing behavior change techniques and how to apply them in design |
|
|
| Data design | Translate the design requirements into a data infrastructure. Setup, implementation, and configuration of patient engagement platform |
|
|
| Clinical knowledge | Basic knowledge of the context and the condition and translating clinical input to design requirements and content items |
|
|
| Copywriting | Tone of voice and tailoring items to patients' needs |
|
|
| Clinical capabilities |
Medical knowledge | In-depth clinical knowledge about the condition at hand and the network to reach out to others when more expert knowledge is required, including knowledge of existing behavior change techniques and how to implement them |
|
| Clinical operational knowledge | Ability to translate clinical practice to useful content. Practical implementation of clinical knowledge into content development and timing of content items in care pathway |
|
|
| Patient understanding | Translating patient needs to content and timing of content, in a way 'bypassing' patient interviews because they know so much about the patients |
|
Discussion
In this section, we wish to further discuss some specific elements of the process that went well and some places where we still see room for improvement for future content creation processes. For this, we look at the broader scope of the future of using content and data in healthcare practices to establish personalized lifestyle coaching. Therefore, we want to take the opportunity to reflect on the separate elements of the process to see where they might be streamlined and automated to create a more fluent flow from a care pathway design to the content on the patient’s device.
Personalized Lifestyle Coaching
Patients with different risk factors require different treatments, which is the level at which we have currently implemented personalization. However, patients with a comparable risk profile might still experience their complaints differently and require a different approach. For example, some patients indicated finding it hard to make time to read the content, while others would have liked to receive even more. Therefore, we believe that further personalization of the care content will be required in the future.
From a technological perspective, we ran into the issue that the degrees of personalization that could be achieved with the available resources were limited. While we would have liked to have patient-specific content tailored to their personal situation, similar to previous work (Jansen et al., 2020), this was difficult to scale up with the current data infrastructure. We faced a situation where creating more personalized content meant creating more versions of the content. While we did this for some situations (i.e., two different care programs for either an internal fitness program or a more extensive program at a specialized facility), this was limited to situations where the information would otherwise be factually incorrect (e.g., stating the wrong location or specialist for procedures). In the current setup, each step of personalization exponentially increases the number of content items that need to be prepared, increasing the workload for the technical and the content development team.
Integration of Care
At this time, we were unfortunately limited to presenting the content items through an online platform only accessible via the patients’ web browser, with the secure but cumbersome sign-in procedure associated with clinical platforms. Ideally, this would be more strongly integrated into the everyday life of the patients and be available on the channel or device they prefer to use. Additionally, data tracking could have been more integrated as in (Jansen et al., 2020), so that the content would be delivered based on data triggers. For example, we could trigger content based on changes in patients’ weight and blood pressure measurements, or even on events (e.g., consult appointments) that are logged in the patient’s EMR. We could also present content based on the patients’ location and at a time of the day that is indicated most convenient for them.
The patient engagement platform and the way it was set up and tailored for this trial could help with further integration of future healthcare. The integrated care approach is a proven strategy in the management of patients with atrial fibrillation (Gallagher et al., 2017). This includes the involvement of a multidisciplinary team of health professionals in the patient’s treatment. When patients are under treatment for different conditions at the same time, different caregivers might naturally not adjust simultaneous treatments to one another because of logistical constraints (e.g., incompatible workflows, location of treatments, availability of specialists). To streamline AF care, we already designed the care pathways for multiple treatments and interventions so that the patient perceives the multiple care procedures and accompanying information as one well-coordinated pathway. Many of the content items or entire care programs from this trial are already reusable for other care pathways. To further improve patient experience and involvement in the future, integrating care programs for comorbidities would make for an even better healthcare experience.
Achieving Patient Goals
One of the key elements of lifestyle coaching in the AF outpatient clinic is to encourage and support the patients in achieving their personal goals. Next to educating patients with personalized information, the clinicians were interested in sending tasks to patients (e.g., to actively consider the food products they consume at home). Tasks like these, too, are facilitated in the Engage platform as scheduled tasks in the care programs. Furthermore, the care provider can order patients to share home measurements (for body weight or blood pressure). Having access to the measurements and activity data around the tasks enables clinicians to prepare for consultation meetings and to activate patients in their lifestyle changes. For example, when their body weight management is not as successful as planned, they can order an extra consultation meeting. Ultimately, the clinicians can then provide more efficient consultations and personalized advice and improve patient involvement. For future work, we see opportunities to make the goals more central to the content that patients receive.
Ethical Considerations
While we see evidence from our preliminary data that patients in the clinical trial are engaging with the created content and we have created the content with the extensive requirements in mind, we find it important to also reflect on how solutions like our platform contribute to a digital divide. Previous research has shown that older adults with lower socio-economic status, as well as a minority status are significantly less likely to use the internet for health information (Yoon et al., 2020). By using an online platform to gain access to health information, the solution directly excludes these individuals, as well as those who have difficulty using digital systems or who do not wish to use connected technology for different reasons (e.g., a cognitive or physical disability). In the ongoing study, inclusion criteria included basic technological literacy, as well as B1 level Dutch literacy. For future work, we wish to highlight the importance of making patient engagement platforms and educational content for patients accessible and inclusive for all patients. This means that further research is required into the barriers that the patients that were presently excluded from the study experience, and how their experience could be improved to meet the same standards as other patients. In practice, the approach to information delivery and lifestyle coaching will continue to require a case-by-case assessment from a healthcare professional. A patient engagement platform as presented in this study will hopefully work for a significant number of patients, but we do not recommend this approach as a one-size-fits-all solution. We encourage designers and clinicians to carefully consider those who might not benefit from these types of solutions and to think about how to keep on providing personal and high-quality care to them, too.
Future Opportunities
In the future, we envision that a content delivery platform like the one being used in the POP trial would be expanded to different hospitals and departments within those hospitals. Some of the care programs used for this trial could directly be reused for other patient groups, and new care programs can easily be added as well. However, having a dedicated content creation workflow is required to help achieve this in different places with different teams. Adapting the care programs to the personal situation of patients could be supported by creating parametric content and facilitating different content formats. Expanding the content to lower-risk populations would also enable preventative use of the content.
Tools to Support Content Creation
The first challenge to tackle would be the manual labor associated with uploading the content to the content platform. This process could be easily automated by letting the platform import an Excel sheet with all necessary data. An online creation platform could also help to keep track of changes to content items and care programs. Sharing the content with other team members online through Miro worked for this project, but we encountered issues with different versions being spread to different members of the team, leading to version conflicts and the associated extra work to streamline versions at the end of the process.
Content and content creation is an opportune area for the design community to research and a promising application area for new specialized tools. Although the content might not always be the core contribution of healthcare platform design, the process of content creation, validation, and delivery must be smooth in all its complexity. This requires a redesign of the platform to better support quickly editing both individual items and the care programs. Additionally, having a review feature within the content management system, rather than a separate platform entirely, would also significantly streamline the validation process.
From a clinical perspective, the patient engagement platform also creates the opportunity for clinicians to directly learn from patient experience. Are patients motivated to read more background information? Do patients interact more with a stress and anxiety module? The way patients will use the digital health platform gives insight into what is perceived as relevant information. Hereby, the clinician can not only provide a more personalized experience for the patient, but future patients can benefit from knowledge and insight from those experiences.
Dynamic Content
An issue that we encountered was the sheer amount of content items to reach the level of personal attention and understanding that the clinicians intuitively offer in face-to-face consultation meetings. We wanted our system to be an extension of that type of interaction, but it was impossible to reach that nuance without the exponential growth of the content database. If content items could be created in a parametric way, where variables are saved that influence the content of the messages, far fewer content items would need to be created and sent. This could lower the burden of treatment for patients as they receive fewer and more relevant notifications, and it could lower the workload of clinicians by taking some of the face-to-face personalization tasks that they currently undertake.
Personalization of Content Delivery
Besides personalizing through offering different care programs and content items, we also see great potential in personalizing the type of content (e.g., the medium, the length of the text, and the tone of voice). In our experience, it was difficult to write content in a way that would be engaging for any kind of audience, and we often went back and forth between a more educational tone that assumed little prior knowledge, and a more academic tone that presented facts based on medical research. As there is a large difference in how people prefer to receive information, based on, e.g., their personality (Sojka & Giese, 2001), we believe that adding this level of personalization could also increase patient engagement and treatment effectiveness. A stronger exploration of the use of visualizations and videos would also help patients with different learning styles to engage more deeply with the content.
Preventative Care
An open issue in providing preventative care is how to deliver it to the right people at the right moment. Especially primary prevention can be difficult as they have usually not had any contact about their health with their doctor yet. It is important to strategically brand the lifestyle content such that it reaches and appeals to a wider audience than only diagnosed patients. Health insurance could play a role in this, or it could be implemented in consumer products rather than patient platforms. However, it is important that the clinical connection is maintained as it is currently difficult for clinicians to rely on consumer products that have not been certified for clinical use and that might not offer comprehensive, secure, and integrative data-sharing features.
Conclusion
In this article, we have reported on the content design and validation process in a novel healthcare solution for AF patients. We documented and reflected in detail on the content creation process and provided in-depth experience reports on various aspects of creation, validation, and delivery. Based on the process, we have identified challenges in our content design process and suggest improvements for future healthcare content creation processes with a focus on personalization. We have identified areas of future interest, with possibilities of streamlining the content creation and validation process. Additionally, we see an opportunity to further personalize content by making it dynamic, tailoring it to more diverse learning styles, and seeking a closer integration with the consumer market to be able to focus on prevention. In an age where content is generated by many different organizations for diverse purposes, we see an emerging role for designers and clinicians to support patients in finding information that is factually correct and relevant to their current health situation.
Endnote
- 1. In this article, we understand ‘healthcare content’ as text, images and videos with the intention to inform, educate and motivate patients about their medical condition and lifestyle.
Acknowledgments
We want to thank all participants of the POP trial and those who participated in extra interviews for their participation. We also want to thank all team members who have contributed to the trial’s design and set-up, specifically, Coen Mulder, Daisy O’Neill, Kathelijn van der Horst, Marleen Hillen, Janne van Kollenburg, Beatrix Hager, Annet de Winter, Peggy de Kievit, and Steven Bressers.
References
- Aldridge, M. D. (2004). Writing and designing readable patient education materials. Nephrology Nursing Journal, 31(4), 373-377.
- Bogers, S., Van Kollenburg, J., Deckers, E., Frens, J., & Hummels, C. (2018). A situated exploration of designing for personal health ecosystems through data-enabled design. In Proceedings of the conference on designing interactive systems (pp. 109-120). ACM. https://doi.org/10.1145/3196709.3196769
- Bruno, C. M. (2020). A content analysis of how healthcare workers use TikTok. Elon Journal of Undergraduate Research in Communications, 11(2), 5-16.
- Cash, P., Gamundi, X. V., Echstrøm, I., & Daalhuizen, J. (2022). Method use in behavioural design: What, how, and why? International Journal of Design, 16(1), 1-21. https://doi.org/10.57698/v16i1.01
- Chatterjee, A., Prinz, A., Gerdes, M., & Martinez, S. (2021). Digital interventions on healthy lifestyle management: Systematic review. Journal of Medical Internet Research, 23(11), Article e26931. https://doi.org/10.2196/26931
- Cherian, T. S., Shrader, P., Fonarow, G. C., Allen, L. A., Piccini, J. P., Peterson, E. D., Thomas, L., Kowey, P. R., Gersh, B. J., & Mahaffey, K. W. (2017). Effect of atrial fibrillation on mortality, stroke risk, and quality-of-life scores in patients with heart failure (from the outcomes registry for better informed treatment of atrial fibrillation [ORBIT-AF]). The American Journal of Cardiology, 119(11), 1763-1769. https://doi.org/10.1016/j.amjcard.2017.02.050
- Chow, C. K., Redfern, J., Hillis, G. S., Thakkar, J., Santo, K., Hackett, M. L., Jan, S., Graves, N., De Keizer, L., Barry, T., Bompoint, S., Stepien, S., Whittaker, R., Rodgers, A., & Thiagalingam, A. (2015). Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: A randomized clinical trial. Jama, 314(12), 1255-1263. https://doi.org/10.1001/jama.2015.10945
- Chung, M. K., Eckhardt, L. L., Chen, L. Y., Ahmed, H. M., Gopinathannair, R., Joglar, J. A., Noseworthy, P. A., Pack, Q. R., Sanders, P., & Trulock, K. M. (2020). Lifestyle and risk factor modification for reduction of atrial fibrillation: A scientific statement from the American Heart Association. Circulation, 141(16), e750-e772. https://doi.org/10.1161/CIR.0000000000000748
- Collings, C., Frates, E. P., & Shurney, D (2022). The time is now for lifestyle medicine: Lesson from lifestyle medicine leaders. American Journal of Lifestyle Medicine, 16(5), 557-561. https://doi.org/10.1177/15598276221088807
- Dinh-Le, C., Chuang, R., Chokshi, S., & Mann, D (2019). Wearable health technology and electronic health record integration: Scoping review and future directions. JMIR Mhealth and Uhealth, 7(9), Article e12861. https://doi.org/10.2196/12861
- Flores, M., Glusman, G., Brogaard, K., Price, N. D., & Hood, L. (2013). P4 medicine: How systems medicine will transform the healthcare sector and society. Personalized Medicine, 10(6), 565-576. https://doi.org/10.2217/pme.13.57
- Forastiere, M., Pietro, G. D., & Sannino, G. (2016). An mHealth application for a personalized monitoring of one’s own wellness: Design and development. In R. J. Howlett & L. C. Jain (Eds.), Smart innovation, systems and technologies (pp. 269-278). Springer. https://doi.org/10.1007/978-3-319-39687-3_26
- Gallagher, C., Elliott, A. D., Wong, C. X., Rangnekar, G., Middeldorp, M. E., Mahajan, R., Lau, D. H., Sanders, P., & Hendriks, J. M. (2017). Integrated care in atrial fibrillation: A systematic review and meta-analysis. Heart, 103(24), 1947-1953. http://dx.doi.org/10.1136/heartjnl-2016-310952
- Groeneveld, B., Melles, M., Vehmeijer, S., Mathijssen, N., Dekkers, T., & Goossens, R. (2019). Developing digital applications for tailored communication in orthopaedics using a research through design approach. Digital Health, 5. https://doi.org/10.1177/2055207618824919
- Hendriks, J. M., De Wit, R., Crijns, H. J., Vrijhoef, H. J., Prins, M. H., Pisters, R., Pison, L. A. F. G., Blaauw, Y., & Tieleman, R. G. (2012). Nurse-led care vs. usual care for patients with atrial fibrillation: Results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. European Heart Journal, 33(21), 2692-2699. https://doi.org/10.1093/eurheartj/ehs447
- Holmlid, S., Mattelmäki, T., Visser, F. S., & Vaajakallio, K. (2015). Co-creative practices in service innovation. In R. Agarwal, W. Selen, G. Roos, & R. Green (Eds.), The handbook of service innovation (pp. 545-574). Springer.
- Jansen, J. M., Niemantsverdriet, K., Burghoorn, A. W., Lovei, P., Neutelings, I., Deckers, E., & Nienhuijs, S. (2020). Design for co-responsibility: Connecting patients, partners, and professionals in bariatric lifestyle changes. In Proceedings of the conference on designing interactive systems (pp. 1537-1549). ACM. https://doi.org/10.1145/3357236.3395469
- Khan, A. M., & Lee, S. W. (2013). Need for a context-aware personalized health intervention system to ensure long-term behavior change to prevent obesity. In Proceedings of the 5th international workshop on software engineering in health care (pp. 71-74). IEEE. https://doi.org/10.1109/SEHC.2013.6602481
- Kelly, M. P., & Barker, M. (2016). Why is changing health-related behaviour so difficult? Public Health, 136, 109-116. https://doi.org/10.1016/j.puhe.2016.03.030
- Van Kollenburg, J., & Bogers, S. J. A. (2019). Data-enabled design: A situated design approach that uses data as creative material when designing for intelligent ecosystems [Doctoral dissertation, Eindhoven University of Technology]. TU/e Repository. https://research.tue.nl/en/publications/data-enabled-design-a-situated-design-approach-that-uses-data-as-
- Van Kollenburg, J., Bogers, S., Rutjes, H., Deckers, E., Frens, J., & Hummels, C. (2018). Exploring the value of parent tracked baby data in interactions with healthcare professionals: A data-enabled design exploration. In Proceedings of the SIGCHI conference on human factors in computing systems (Article No. 297). ACM. https://doi.org/10.1145/3173574.3173871
- Kornej, J., Börschel, C. S., Benjamin, E. J., & Schnabel, R. B. (2020). Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circulation research, 127(1), 4-20. https://doi.org/10.1161/CIRCRESAHA.120.316340
- Kramer, L. L., Ter Stal, S., Mulder, B. C., de Vet, E., & van Velsen, L. (2020). Developing embodied conversational agents for coaching people in a healthy lifestyle: Scoping review. Journal of Medical Internet Research, 22(2), Article e14058. https://doi.org/10.2196/14058
- Kreps, G. L. (2014). Evaluating health communication programs to enhance health care and health promotion. Journal of Health Communication, 19(12), 1449-1459. https://doi.org/10.1080/10810730.2014.954080
- Kuwabara, A., Su, S., & Krauss, J. (2020). Utilizing digital health technologies for patient education in lifestyle medicine. American Journal of Lifestyle Medicine, 14(2), 137-142. https://doi.org/10.1177/1559827619892547
- Lane, D. A., McMahon, N., Gibson, J., Weldon, J. C., Farkowski, M. M., Lenarczyk, R., Watkins, C. L., Dilaveris, P., Caiani, E. G., & Potpara, T. S. (2020). Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: A systematic review. EP Europace, 22(10), 1567-1578. https://doi.org/10.1093/europace/euaa269
- Li, Y., Pan, A., Wang, D. D., Liu, X., Dhana, K., Franco, O. H., Kaptoge, S., Angelantonio, E. D., Stampfer, M., Willett, W. C., & Hu, F. B. (2018). Impact of healthy lifestyle factors on life expectancies in the US population. Circulation, 138(4), 345-355. https://doi.org/10.1161/CIRCULATIONAHA.117.032047
- Little, D. (2006). The common European framework of reference for languages: Content, purpose, origin, reception and impact. Language Teaching, 39(3), 167-190. https://doi.org/10.1017/S0261444806003557
- Lovei, P., Deckers, E., Funk, M., & Wensveen, S. (2020). The marios and luigis of design: Design plumbers wanted! In Companion publication of the conference on designing interactive systems (pp. 197-201). ACM. https://doi.org/10.1145/3393914.3395898
- Ma, J., Rosas, L. G., & Lv, N. (2016). Precision lifestyle medicine: A new frontier in the science of behavior change and population health. American Journal of Preventive Medicine, 50(3), 395-397. https://doi.org/10.1016/j.amepre.2015.09.035
- Mahmud, A. J., Olander, E., Eriksén, S., & Haglund, B. J. (2013). Health communication in primary health care: A case study of ICT development for health promotion. BMC Medical Informatics and Decision Making, 13(1), Article 7. https://doi.org/10.1186/1472-6947-13-17
- Manfredini, E. (2021). Providing health information on social media: What is the limit for medical students? International Journal of Medical Students, 9(1), 94-95. https://doi.org/10.5195/ijms.2021.979
- Madathil, K. C., Rivera-Rodriguez, A. J., Greenstein, J. S., & Gramopadhye, A. K. (2014). Healthcare information on YouTube: A systematic review. Health Informatics Journal, 21(3), 173-194. https://doi.org/10.1177/1460458213512220
- Mayo Clinic (2022). Cardiac ablation. https://www.mayoclinic.org/tests-procedures/cardiac-ablation/about/pac-20384993
- McCabe, P. J. (2011). What patients want and need to know about atrial fibrillation. Journal of Multidisciplinary Healthcare, 4, 413-419. https://doi.org/10.2147/JMDH.S19315
- McCabe, P. J., Kumbamu, A., Stuart-Mullen, L., Hathaway, J., & Lloyd, M. (2020). Exploring patients’ values and preferences for initial atrial fibrillation education. Journal of Cardiovascular Nursing, 35(5), 445-455. https://doi.org/10.1097/JCN.0000000000000716
- Menheere, D., Lallemand, C., Faber, I., Pepping, J., Monkel, B., Xu, S., & Vos, S. (2019). Graceful interactions and social support as motivational design strategies to encourage women in exercising. In Proceedings of the halfway to the future symposium (Article No. 20). ACM. https://doi.org/10.1145/3363384.3363404
- Molapo, M., & Marsden, G. (2013). Software support for creating digital health training materials in the field. In Proceedings of the 6th international conference on information and communication technologies and development (pp. 205-214). ACM. https://doi.org/10.1145/2516604.2516632
- Niedderer, K., Clune, S., & Ludden, G. (2017). Design’s intrinsic relationship with change and its challenges for the 21st century. In K. Niedderer, S. Clune, & G. Ludden (Eds.), Design for behaviour change (pp. 9-15). Routledge. https://doi.org/10.4324/9781315576602
- Noortman, R., Funk, M., Andersen, K., & Eggen, B. (2021). What would margaret atwood do? Designing for ustopia in HCI. In Proceedings of the 24th international academic mindtrek conference (pp. 72-80). ACM. https://doi.org/10.1145/3464327.3464344
- Noortman, R., Lovei, P., Funk, M., Deckers, E., Wensveen, S., & Eggen, B. (2022). Breaking up data-enabled design: Expanding and scaling up for the clinical context. AI EDAM, 36, Article e19. https://doi.org/10.1017/S0890060421000433
- Pathak, R. K., Middeldorp, M. E., Meredith, M., Mehta, A. B., Mahajan, R., Wong, C. X., Twomey, D., Elliott, A. D., Kalman, J. M., Abhayaratna, W. P., Lau, D. H., & Sanders, P. (2015). Long-term effect of goal-directed weight management in an atrial fibrillation cohort: A long-term follow-up study (LEGACY). Journal of the American College of Cardiology, 65(20), 2159-2169. http://dx.doi.org/10.1016/j.jacc.2015.03.002
- Pina, L. R., Ramirez, E., & Griswold, W. G. (2012). Fitbit+: A behavior-based intervention system to reduce sedentary behavior. In Proceedings of the 6th international conference on pervasive computing technologies for healthcare and workshops (pp. 175-178). IEEE. https://doi.org/10.4108/icst.pervasivehealth.2012.248761
- Salmasi, S., Kwan, L., MacGillivray, J., Bansback, N., De Vera, M. A., Barry, A. R., Harrison, M. J., Andrade, J., Lynd, L. D., & Loewen, P. (2019). Assessment of atrial fibrillation patients’ education needs from patient and clinician perspectives: A qualitative descriptive study. Thrombosis Research, 173, 109-116. https://doi.org/10.1016/j.thromres.2018.11.015
- Sanders, E. B. N., & Stappers, P. J. (2008). Co-creation and the new landscapes of design. Co-design, 4(1), 5-18. https://doi.org/10.1080/15710880701875068
- Sanz, M. F., Acha, B. V., & García, M. F. (2021). Co-design for people-centred care digital solutions: A literature review. International Journal of Integrated Care, 21(2), Article 16. https://doi.org/10.5334/ijic.5573
- Seo, W., Buyuktur, A. G., Verma, S., Kim, H., Choi, S. W., Sedig, L., & Park, S. Y. (2021). Learning from healthcare providers’ strategies: Designing technology to support effective child patient-provider communication. In Proceedings of the SIGCHI conference on human factors in computing systems (Article No. 660). ACM. https://doi.org/10.1145/3411764.3445120
- Singleton, A., Raeside, R., Partridge, S. R., Hayes, M., Maka, K., Hyun, K. K., Thiagalingam, A., Chow, C. K., Sherman, K. A., Elder, E., & Redfern, J. (2021). Co-designing a lifestyle-Focused text message intervention for women after breast cancer treatment: Mixed methods study. Journal of Medical Internet Research, 23(6), Article e27076. https://doi.org/10.2196/27076
- Slattery, P., Saeri, A. K., & Bragge, P. (2020). Research co-design in health: A rapid overview of reviews. Health Research Policy And Systems, 18(1), Article 17. https://doi.org/10.1186/s12961-020-0528-9
- Smith, S., & Ashby, M. (2020). How to future: Leading and sense-making in an age of hyperchange. Kogan Page.
- Sojka, J. Z., & Giese, J. L. (2001). The influence of personality traits on the processing of visual and verbal information. Marketing Letters, 12(1), 91-106.
- Soroya, S. H., Farooq, A., Mahmood, K., Isoaho, J., & Zara, S. (2021). From information seeking to information avoidance: Understanding the health information behavior during a global health crisis. Information Processing & Management, 58(2), Article 102440. https://doi.org/10.1016/j.ipm.2020.102440
- St. Amant, K. (2015). Aspects of access: Considerations for creating health and medical content for international audiences. Communication Design Quarterly Review, 3(3), 7-11. https://doi.org/10.1145/2792989.2792990
- Vermeer, J., Vinck, T., de Louw, B., Slingerland, S., van’t Veer, M., Regis, M., ... & Dekker, L. (2023). Improving outcomes of AF ablation by integrated personalized lifestyle interventions: rationale and design of the prevention to improve outcomes of PVI (POP) trial. Clinical Research in Cardiology, 112(6), 716-723. https://doi.org/10.1007/s00392-023-02185-5
- Villalobos-Zúñiga, G., & Cherubini, M. (2020). Apps that motivate: A taxonomy of app features based on self-determination theory. International Journal of Human-Computer Studies, 140, Article 102449. https://doi.org/10.1016/j.ijhcs.2020.102449
- Wolin, K. Y., Glynn, R. J., Colditz, G. A., Lee, I. M., & Kawachi, I. (2007). Long-term physical activity patterns and health-related quality of life in U.S. women. American Journal of Preventive Medicine, 32(6), 490-499. https://doi.org/10.1016/j.amepre.2007.02.014
- World Health Organization. (2010). A healthy lifestyle - WHO recommendations. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations
- Yoon, H., Jang, Y., Vaughan, P. W., & Garcia, M. (2020). Older adults’ internet use for health information: Digital divide by race/ethnicity and socio-economic status. Journal of Applied Gerontology, 39(1), 105-110. https://doi.org/10.1177/0733464818770772