Towards A Framework for Holistic Contextual Design for Low-Resource Settings
Clara B. Aranda-Jan 1,*, Santosh Jagtap 2, and James Moultrie 1
1 Institute for Manufacturing, Engineering Department, University of Cambridge, Cambridge, UK
2 Indian Institute of Technology Bombay, India and School of Industrial Design, Faculty of Engineering, Lund University, Lund, Sweden
Healthcare inequality is ubiquitous globally, but the effects are most striking in low-resource settings. In these settings, current methods for the design of medical devices are failing to address specific needs. The associated publications rarely describe how the context was studied at the front-end of design. There is a latent need for a holistic contextual framework for guiding the design decision-making process for devices in these complex contexts. We present results from a systematic literature review and expert interviews that informed the development of a framework for contextualized design for low-resource settings. The contextual factors identified are described and compared for different types of medical devices. This taxonomical framework aims to guide designers towards gaining a better understanding of the context of use when designing products for global challenges in low-resource settings.
Keywords – Design for Development, Context of Use, Frugal Innovations, Developing Countries, Healthcare.
Relevance to Design Practice – The study provides a comprehensive contextual framework to support design practitioners to gather, compile and analyse contextual information for the design of products for low-resource settings. The proposed framework is broad in scope to highlight under-appreciated and often neglected aspects of context, to aid decision-making at the earliest stages of product design and to avoid possible failures in “the last mile” of getting a product to market.
Citation: Aranda-Jan, C. B., Jagtap, S., & Moultrie, J. (2016). Towards a framework for holistic contextual design for low-resource settings. International Journal of Design, 10(3), 43-63.
Received January 11, 2016; Accepted October 27, 2016; Published December 31, 2016.
Copyright: © 2016 Aranda-Jan, Jagtap, & Moultrie. Copyright for this article is retained by the authors, with first publication rights granted to the International Journal of Design. All journal content, except where otherwise noted, is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 2.5 License. By virtue of their appearance in this open-access journal, articles are free to use, with proper attribution, in educational and other non-commercial settings.
*Corresponding Author: cba26@cam.ac.uk
Clara B. Aranda-Jan is a Biomedical Engineer from Tecnologico de Monterrey, Mexico. She obtained an MPhil in Engineering for Sustainable Development from the University of Cambridge. She has work and research experience in the public health and sustainability fields. She was a consultant for the World Health Organization (Switzerland) and UNICEF Supply Division (Denmark) where she worked on medical devices for maternal, newborn and child health, and the impact of the lack of electricity in rural healthcare facilities. She worked for the Institute of Public Health at Heidelberg University as a researcher assistant, analysing factors for adequate implementation of mHealth projects in Africa and the impact of climate change on malnutrition. Clara is now studying for her PhD at the University of Cambridge in the Institute for Manufacturing, where she is also the Deputy Director of the iTeams programme.
Santosh Jagtap is Assistant Professor at the Indian Institute of Technology Bombay and is also affiliated with Lund University, Sweden. He completed his PhD in Engineering Design at the University of Cambridge, UK and an M.Des. in Product Design at the Indian Institute of Science, Bangalore. He has previously worked at Delft University of Technology and Lund University. His research focus is on understanding and improving design processes in a variety of contexts. He has published his research in journals such as Design Studies, Research in Engineering Design, Journal of the American Society for Information Science and Technology, International Journal of Design Creativity and Innovation, and International Journal of Sustainable Society.
James Moultrie has a background in industrial design and mechanical engineering. Before joining academia, James had an industrial career, where he was responsible for numerous projects, including metrology instruments and lenses for the movie industry for which he was awarded a ‘Scientific and Technical Academy Award external link’ (Oscar) in 2000. He is well known for his research investigating the ‘value of design’, including the ‘Design Scoreboard’ project, which developed an original comparison of national design capabilities. He was also a member of a European project, which established protocols for measuring the economic value of design. He is interested in design for manufacture and regularly works with companies to improve design for assembly. Current work includes activity exploring ‘Design for Additive Manufacturing’.
Introduction
There are unacceptable health inequalities around the world. The conditions in which people are born, work and live, and the systems put in place to support healthy lifestyles determine many of these inequalities (The World Bank, 2015). Many argue that these socially determined inequalities are avoidable if these conditions are designed appropriately to reduce the risk of illness and disease (Manzini, 2014; Waddell, 2012). It is by design that we might begin to tackle the intractable global challenges of health inequity, poverty alleviation and development (Lawrence, 2014; Mulgan, Tucker, Ali, & Sanders, 2007).
Designing for Global Health Challenges
The design of medical devices has failed to deliver effective and efficient products to low-resource settings (LRSs). When visiting a healthcare facility in a developing country, the poor condition of healthcare technologies is evident. Facilities often contain obsolete or totally dysfunctional equipment (Free, 2004; Howitt et al., 2012; Malkin, 2007a, 2007b; Sinha & Barry, 2011). In other cases, expensive equipment lies dormant because there are inadequate skills and materials for its use, repair or maintenance. To adequately address global health challenges faced in LRSs, medical devices need to be designed to be sympathetic with the local conditions and context. Technologies need “to meet the needs of the world’s poorest people” (Howitt et al., 2012, p.509) and be “good enough to meet the demands of customers who could not afford state-of-the-art technology” (Ibid., p.528). These technologies cannot merely replicate successful ones in the developed world, but should instead be designed to be cognisant of the local context (Arasaratnam & Humphreys, 2013; Free, 2004; Niemeier, Gombachika, & Richards-Kortum, 2014).
However, success stories are limited in the development of medical devices for LRSs (Sinha & Barry, 2011). Recently, numerous technology-based projects for global health have been funded, but information on their effectiveness is limited. Most of the medical devices designed for LRSs have been designed by people in developed countries and few have gone beyond being mere university projects (Garrett, 2007; Jagtap, Larsson, Hiort, Olander, Warell, & Khadilkar, 2014; Sienko, Sarvestani, & Grafman, 2013). The disconnection between the designer, her understanding of the context and the reality in the context might contribute to this systemic design failure. Challenges in understanding the context of use of products in LRSs have been reported as potential causes of failed designs (Free, 2004; Wood & Mattson, 2016). Indeed, developing appropriate medical devices can be more challenging when designing for LRSs than for developed countries (Bergmann, Noble, & Thompson, 2015). A reason behind this is that the task of gathering and interpreting contextual information in LRSs is hard, time-consuming and costly, particularly for less experienced designers (Castillo, Diehl, & Brezet, 2012; Mohedas, Daly, & Sienko, 2015).
Accurate collection of user requirements and contextual information has proven to be highly valuable in medical device design (Martin, Clark, Morgan, Crowe, & Murphy, 2012). When designing for LRSs, designers must have a deep understanding of the context of use at an early stage of device design (Castillo et al., 2012; Donaldson, 2006; Nakata & Weidner, 2012; Rodriguez, Diehl, & Christiaans, 2006). The inherent complexity of these contexts demands that designers devote a considerable amount of time to scoping the problem, gathering contextual information and defining design requirements (Jagtap et al., 2014; Jagtap, Larsson, Hjort af Ornäs, Olander, & Warell, 2013b). Often, however, designers overlook this complexity and consequently, products are poorly designed according to expected rather than experienced contexts (Donaldson, 2006).
A good definition of context is essential if a product is to be successful in the market (Mohedas, Daly, & Sienko, 2014a, 2014b; Nemoto, Uei, Sato, & Shimomura, 2015). Few frameworks exist to support designers, largely unfamiliar with the notion of context, to characterize the context in the design process of products for LRSs (Green, 2005; for instance, Green, Linsey, Seepersad, Wood, & Jensen, 2006). To our knowledge, no framework has focused on medical devices for LRSs. Recommendations for developing medical devices for LRSs are relatively common in the literature, but little guidance is available for designers regarding context and how to study context (Free, 2004; Malkin, 2007b; Wood & Mattson, 2014).
Given the importance of context, it is surprising that the literature on medical devices for LRSs is limited in the definitions of the term. The World Health Organization [WHO] (2010) describes the context as the “aggregate of factors that influence the use of medical devices” (p.5) such as the characteristics of the healthcare facilities, the supply of devices, the organizational structure for the provision of care and the expectations of healthcare staff for the device. Likewise, Gauthier, Cruz, Medina, and Duke (2013) suggest that designing medical devices for LRSs should consider the characteristics of the device and the setting. The setting, in the view of Gauthier et al. (2013), includes the facilities available, population dynamics and conditions for implementation in the target country (standards, intellectual property, import/export policies).
A broad range of political, social, cultural and environmental factors determine the use and appropriateness of devices in LRSs (Bergmann et al., 2015; Free, 2004). Solely designing for a setting—mainly being the physical environment—has not ensured that devices satisfy the needs of the context. The compelling complexity of any LRS and their healthcare systems suggest the need for a new holistic approach to the context for designing medical devices for these settings. Contextual factors thus need to be holistically explored by designers. It is clear that definitions of context for medical devices for LRSs are falling short and must go beyond those provided by the WHO (2010) and Gauthier et al. (2013).
Definitions of the “Context of Use”
In the field of design, context of use has been given a number of different definitions (Hekkert, & van Dijk, 2011; Nemoto et al., 2015; Rosenman & Gero, 1998; Schifferstein & Hekkert, 2008; Stappers, Hekkert, & Keyson, 2007). In the Glossary of Human Computer Interaction, Soegaard and Dam (n.d.) describe the context of use as the set of “actual conditions under which a given artefact/software product is used, or will be used in a normal day to day working situation” [online]. Nemoto et al. (2015) define context as a “set of spatial-temporal elements related to the person or product” (p.43). Hekkert, and van Dijk (2011) consider that a “seemingly endless number of mechanisms […] co-determine what people are and need, and what products could and should provide” (p.15). These factors constitute the “context of use” of a product. Deconstructing or understanding this context layer is fundamental to the design process to characterise the product-user interactions as a pre-cursor to developing a design solution. The context layer does not describe the technical dimensions of a product, but rather contains ideas, views or other considerations about people, their lives, culture, nature, society and technology.
Rosenman and Gero (1998) propose a different perspective. They suggest that there are three environments that are fundamental for formulating a design problem statement and conceptualizing a solution for the design of an object. These are the natural environment, the human socio-cultural environment and the artefact’s techno-physical system. Objects are intentionally designed to serve a purpose. The purpose by the socio-cultural and the natural environments in which humans interact. Humans interpret the world within these environments and design needs are defined by both, whether generated by the physical world or perceived according to values and goals within the socio-cultural environment. The object is designed to function (serve a purpose) based on the behaviour given by the specific structure of the object. The object creates an artificial techno-physical environment. These three types of environments define the context by interacting with each other and the artifact acquires meaning through this context (Rosenman & Gero, 1998; Schifferstein & Hekkert, 2008).
For human-centered design, Maguire (2001) proposes that the analysis of the context should include the characteristics of the user, the characteristics of the task and the operating environment, both physical and organizational. Methods such as ‘contextual analysis’ will help to specify and evaluate user requirements. The context is the background against which design is taking place and provides an understanding of a product, its usability and safety. Specific to medical devices, the usability standard ISO/IEC 62366-1:2015 defines context in a more pragmatic way as the “actual conditions and settings in which users interact with the medical device” (Advancing Safety in Healthcare Technology, 2015, p. 11).
These definitions make it possible to extract three essential elements that define what the context of use means to designers. Firstly, the context of use is about the users. Users have certain characteristics (e.g., background, beliefs) that set the expectations of what the product is and how it achieves its goals, effectively and efficiently. For a product to achieve the intended goals, the user and the product interact with each other. The daily product-user interactions and usability define how the user experiences the product. Hence, the second element of the context of use is the interactions. Finally, interactions occur within a physical environment or setting (e.g., nature, workplace) as defined by the product-user interactions. The context of use is, therefore, the frame of reference in which a product interacts with and is fully understood by users.
Better understanding the context of use is a much broader activity than just establishing product requirements. In design practice, however, methodological supports are lacking for this task (Braun, Benedict, Wendler, & Esswein, 2015). For medical devices, a review by Alexander and Clarkson (2000a) showed little practical guidance exists for designers to verify and validate design requirements. Taxonomic tools are proven supports for the collection, compilation, organization and interpretation of information at any stage in the design process (Manzini & Coad, 2015). Holistic taxonomical frameworks support designers throughout the design process to conceptualize, assess, develop, refine and implement their ideas in solving design problems (Roser & Walker, 2014).
This paper argues that a holistic framework may support the need to capture the complexity of the context of use of medical devices in LRSs. The framework aims to serve as a taxonomical structure to help designers unfamiliar with these settings to better explore, capture and document contextual information when designing medical technologies for LRSs.
Scope
For this study, we defined medical devices based on the definition by the Food and Drug Administration (FDA) and the EU, as “articles manufactured specifically for diagnostics, monitoring, treatment, or modification of the human body, that are not solely pharmaceutical goods” (Moultrie, Sutcliffe, & Maier, 2015, p. 364). Due to our interest in hardware design, medicines, devices for home-care, vaccines, in-vitro diagnosis, mobile phones or other telecommunication systems applied to healthcare (mHealth or eHealth) sit outside our research scope. The term low-resource setting refers to a resource-constrained (human, economic and environmental) area, rural or urban, with limited infrastructure or basic services in a low- or middle-income country (LMIC) as defined by the World Bank. Used interchangeably, the terms engineer or designer describe an individual in the field of design of medical devices for LRSs, including product designers, engineers, innovators, students and researchers.
Aims and Methods
This study seeks to develop a holistic contextual framework for investigating and characterising factors defining the context of use of medical devices in LRSs. A qualitative approach was used to identify the contextual factors and a quantitative approach to evaluate their relevance.
Data Collection and Extraction
Systematic Literature Review
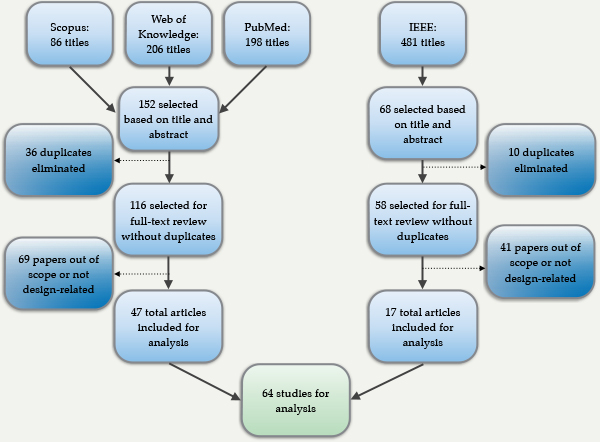
A systematic literature review was undertaken using sources from Pubmed, Scopus, Web of Knowledge and the IEEE Xplore Library for the proceedings from the Global Humanitarian Technology Conference and the Appropriate Health Technologies for Developing Countries Conference. Using Boolean operators AND and OR, the keywords medical devices, medical equipment, low-resource settings, developing countries and low-income country were combined. Only articles in English were included.
The literature searches resulted in a total of 971 titles from all the databases. The criteria for including studies, based on title and abstract review, were:
- the study should refer to a medical device within the research scope, and
- the design should target low-resource settings.
- From the searches, 174 abstracts satisfied the criteria for full-text review, after removing 46 duplicates. Papers to be included in the full-text analysis were selected based on a third inclusion criterion:
- the study should refer to the design process for the device being described, so that the case can be examined for content relating to the designers’ understanding of context.
From the full-text review, 110 papers did not mention a stage in the product development process and were excluded. Hence, 64 journal articles were analysed. The Appendix 1 shows the decision process.
Expert Interviews
Additionally, semi-structured interviews were conducted with experts on designing medical devices for LRSs. A list of projects was compiled from: (1) presenters at WHO’s Global Forum of Medical Devices in November 2013 in Geneva; (2) ASME presentations of appropriate medical devices in 2011; (3) Medical devices published in Appropedia (Sienko et al., 2013); (4) WHO’s Compendium of appropriate medical devices; and (5) the Cooper-Hewitt Museum conference on Design for the other 90%.
When reviewing the projects, 191 medical devices were within the scope of the present study. This list of devices was filtered in two ways. Firstly, only commercially available devices were selected. Secondly, in order to understand perceptions of context, devices designed by people in developed countries were chosen. We worked with the assumption that designers in developed countries are less familiar with the context, but we acknowledge that this assertion could be contested. We consider that this filtering highlights particularly interesting experiences from the interviewees and allows us to understand their coping experiences in the process of learning about the context. Seventy devices resulted from these filters. From this list, organizations with more than one device were considered to have experts in the field. Fourteen organizations satisfied this criterion. We thus identified 34 potential interviewees after obtaining contact details from these organizations via academic publications, organizations’ websites or LinkedIn.
The potential participants were contacted by email. A first invitation was sent introducing the research. A follow-up email was sent to those who did not reply after two weeks. In only one case, a phone call was made before the second email to confirm the interviewee’s email address.
Interview Process
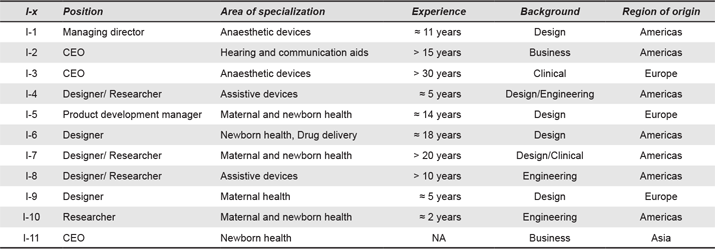
Eleven semi-structured interviews were conducted between February and July 2014. Table 1 describes the characteristics of the interviewees. Since interviewees were located in different parts of the world, interviews were conducted by telephone or Skype. Interviewees were informed that their anonymity will be rigorously maintained. Verbal consent was obtained from each of the participants at the beginning of the interview. Interviews lasted an average of 45 mins and included questions about project description (e.g., idea, motivation), the role of the interviewee in the project, the appropriateness of the device to LRSs, tools used to identify the need, validation of the design and the interviewee’s perception of the LRSs context. Interview recordings were transcribed verbatim using MAXQDA11 for Mac and edited to ensure the participants’ anonymity.
Table 1. Details of interviewees’ positions, device discussed during the interview and years of experience in the field (same or similar technologies).
Data Management
All digital documents were imported to MAXQDA 11 and sets were created according to the type of device and type of data (literature or interview).
Data Analysis
Qualitative Analysis
In vivo and descriptive coding were used to analyse evidence from the literature and interview transcripts (Saldana, 2013). In vivo coding is the process of extracting text as found in the qualitative data record. Descriptive coding is the process of summarizing in a word or short phrase the meaning of the text extracted, requiring some interpretation. When a description of the context was identified, the fragment of text was highlighted and assigned a code. We refer to this fragment of text as a coded segment. Although we had an initial idea of the possible code categories based on literature review (e.g., technical, social, etc.), the factors were allowed to emerge from the data itself. Once all of the studies and transcripts were coded (in vivo and descriptive coding), a second iteration of coding was conducted.
The coded segments were grouped according to the similarity and meaning of the description using focused coding (Saldana, 2013). This procedure helped to identify a common ground for the first level of sub-codes—a contextual factor—that included several coded segments with similar meaning. For instance, interviewee I-3 mentioned: “It was 52ºC in [the operating] theatre. Well, if you put an ordinary anaesthetic machine into that, it is just not going to function.” This segment was initially coded as High Temperature. Similarly, Sharp (1994) mentions that “it is customary in India to remove one’s shoes on entering the house—or temple or mosque—and, in a hot climate, it is very much comfortable to wear sandals rather than closed shoes” (p.208). This segment of text was coded with multiple segments one of which was Hot Climate. During the second iteration of coding, Sharp’s and I-3’s segments would both appear in under a code named Temperature. Sharp’s piece of text would also appear under the code Religious and cultural beliefs. That means that a single piece may contain a description of different contextual factors; in Sharp’s example these are Temperature and Religious and cultural beliefs. Table 2 lists some examples of coded segments.
Table 2. Examples of coded segments.

Contextual factors were grouped into Category of Factors, resulting in a two-layered coded system: code (Category of Factors) and sub-code (Contextual Factor). Using the Code Matrix function, the coded segments were extracted and the coherence of the grouping system was manually verified for each coded segment, sub-code and code.
Quantitative Analysis
To build the framework, we used the Mixed-methods function from MAXQDA to measure the frequency of each Contextual Factor by counting the number of coded segments in the set of documents (literature studies and interview transcripts). The Multiple-code option was used to count this frequency. By using the multiple-code function, the software assumes that the sub-codes are not exclusive of each other and counts the frequency of multiple sub-codes that have been assigned to coded segments in a file. Using the example above for the coded segment from Sharp (1994), the multiple-code function will count the segment twice, once for Temperature and once for Religious and cultural beliefs. Similarly, if another excerpt of text refers to Temperature again, that will be counted giving a count of two to Temperature in the total count in Sharp’s text. Frequencies for each code (Category of Factors) and sub-code (Contextual Factor) were exported to Microsoft Excel to compare the factors across all types of medical devices.
Results and Analysis
Overview of the Types of Medical Devices
Initially, the journal articles (henceforth, also referred as design cases or studies) and interviews were categorized into 23 types of medical devices. After coding, five types of devices lacked contextual information and, therefore, were not analysed further (autoclave, non-pneumatic anti-shock garment, orthopaedic devices, dialysis machine and light source device for otoscope/eye scope). The remaining devices, designed with reference to some aspect of context and aimed at LRSs include anaesthesia machines, neonatal care devices (incubators, phototherapies and continuous positive airway pressure [CPAP] ventilators), assistive devices (hearing aids and prosthetics), patient monitoring devices (pulse oxymetry and blood pressure), patient transportation, suction devices, devices for surgery and surgical support, devices for drug delivery, waste incinerators and devices for anthropometric measurements (scales, mid-upper arm circumference [MUAC] measurement band for diagnosing of malnutrition).
Developing A Holistic Contextual Framework
The studies analysed described the context inconsistently, with some authors providing more detailed descriptions than others. The studies tended to describe the context in a nonspecific way, mentioning aspects common to several LRSs, or any other context. For instance, Malkin and Anand (2010) mentions that “most phototherapy devices are too expensive for developing world hospitals, and the bulbs have a lifespan that is very short” (p. 38, coded as Availability of spare parts). Only a few studies referred to the characteristics specific to a place, country or healthcare facility. An example is given by Edwards (2008), who describes in detail the characteristics of the hospital and the community in Malawi where the Baoband project was implemented. Interviewees described the context more specifically, based on their own personal experiences. For instance, I-1 said:
I was in an operating room in Ethiopia that had nine broken anaesthesia machines. And no functioning machines, before we showed up. (I-1, coded as Functionality of donated equipment)
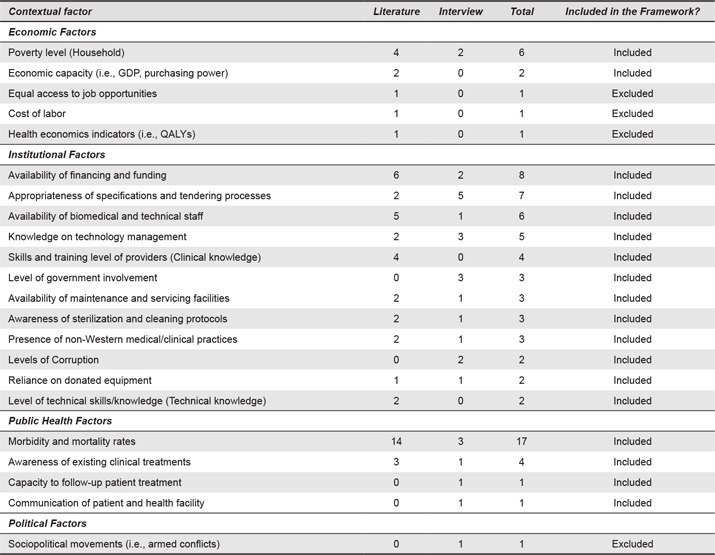
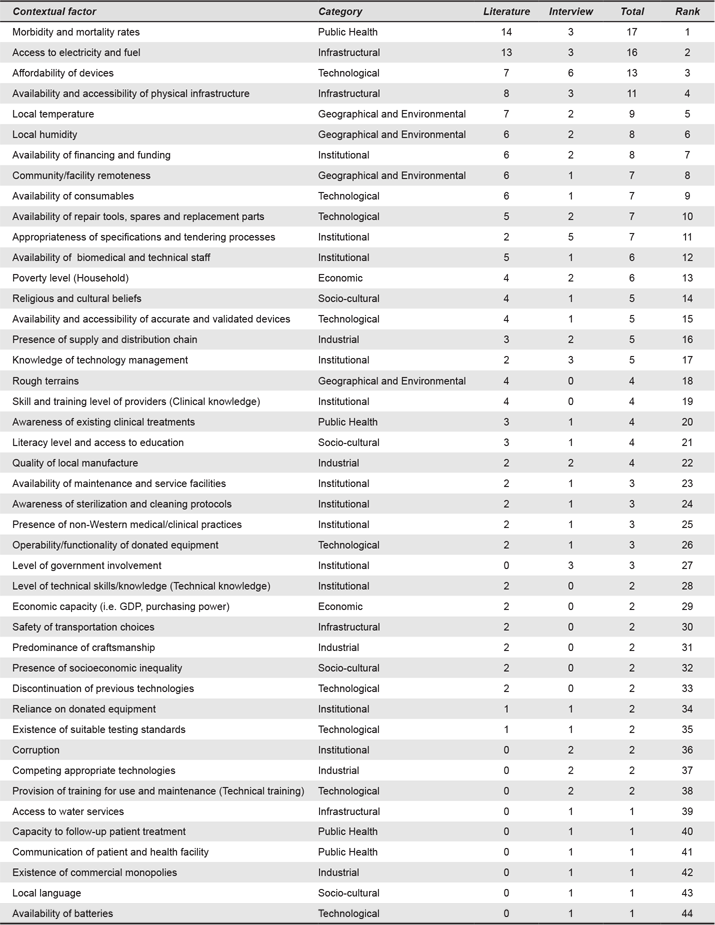
The factors identified aim to cover a broader understanding of the context, a description of the user, setting and interactions. A total of 56 contextual factors were identified (from coded segments of text from transcripts and studies, N = 290) and classified in nine categories (as listed in Table 3): Geographical and Environmental Factors (number of coded segments for this category, n = 31), Institutional Factors (n = 68), Economic Factors (n = 12), Infrastructural Factors (n = 47), Public Health Factors (n = 30), Political Factors (n = 1), Manufacturing and Industrial Factors (n = 23), Socio-cultural Factors (n = 25) and Technological Factors (n = 53).
Table 3. Classification of categories of the holistic contextual framework.
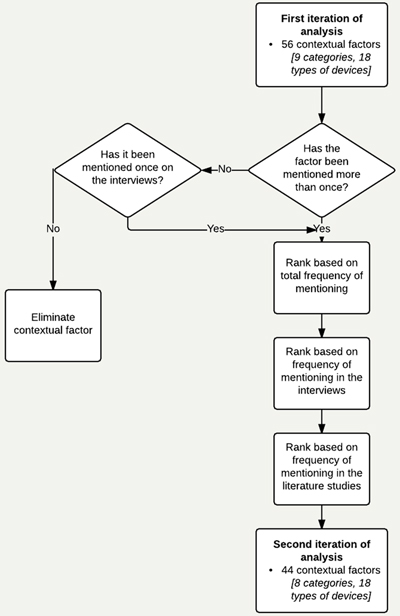
There was a large difference in the frequency of each contextual factor, some of which were mentioned only once and others of which were mentioned more than 15 times. To select the most relevant contextual factors, a second iteration of analysis was conducted. Contextual factors were ranked by relevance, based on a) total frequency, b) frequency of mention during interviews and c) frequency in literature cases. Factors cited once in the studies were excluded, whereas factors mentioned by interviewees once were included. This decision was made based on the idea to build a tool for design practitioners. Experts have first-hand experience of how the context influences success or failure of a device and can give valuable post-mortem knowledge that could be used for pre-mortem assessment of designs during scoping studies. As such, we prioritized designers’ experience, particularly their design practice, over structurally written texts to the demands of academic publications. The second analytical iteration gave 44 contextual factors (N = 205). The decision process is shown in Appendix 2 and Appendix 3 shows the factors ranked. The following sections present the categories of the holistic contextual framework.
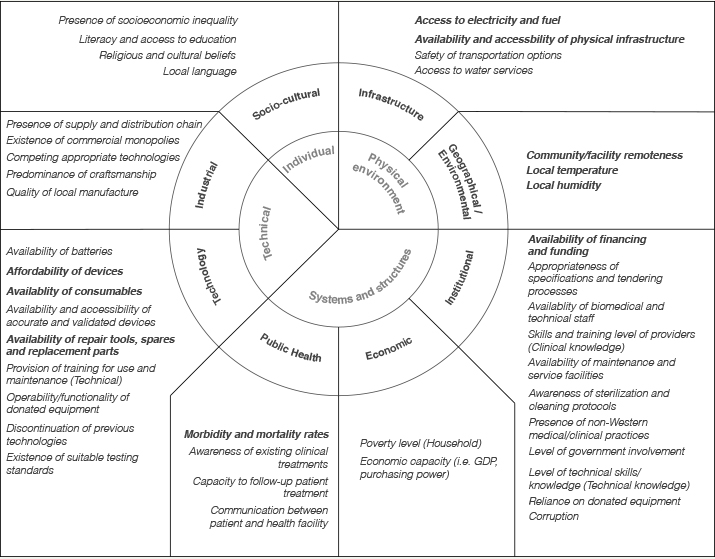
Figure 1. Overview of contextual categories. The most frequently mentioned factors are highlighted in bold.
Technical: Industrial and Technological Factors
Medical devices are designed to fulfil specific needs, for example, those concerning a treatment, diagnosis or prevention of a particular disease or illness. The early design stages will focus on identifying the technical criteria to satisfy that need. Traditional approaches to designing medical devices usually start with the identification of the system requirements, a rather techno-centric approach (Alexander & Clarkson, 2000a, 2000b, 2002; Chaturvedi, Logan, Narayan, & Kuttappa, 2015; Le Cocq, 1987). Medina, Wysk, and Okudan Kremer (2011) and Alexander and Clarkson (2000a) provide reviews of these traditional approaches to the design of medical devices. Although in developed countries good design principles exist for medical devices (i.e., DfX methods, Design for Manufacturing and Assembly, Design for Reliability, Design for Usability), most of these principles are often not applicable or conflict with the actual conditions in LRSs (Nimunkar, Baran, Van Sickle, Pagidimarry, & Webster, 2009; Wood & Mattson, 2014).
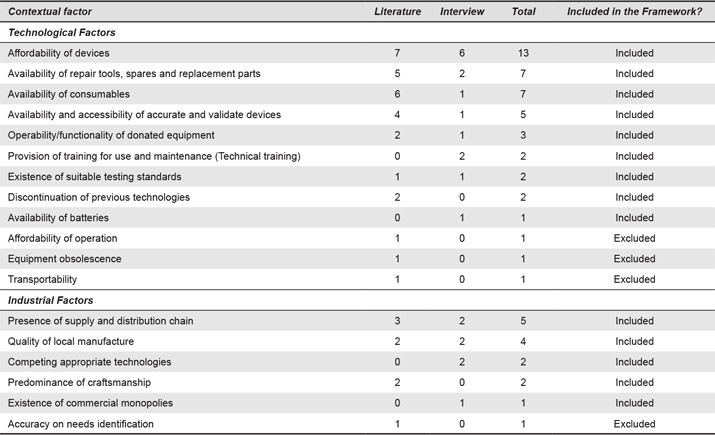
Of the total number of coded segments, these factors were divided into technological factors (n = 42) and industrial factors (n = 14). Technological factors refer to the design requirements that drive the technical aspects of the device and elements needed for adequate operability and functionality of the device. For instance, while the traditional good design practice ensures that medical devices satisfy their intended purpose and commercial aspects, when designing devices for LRSs, elements such as access to spare parts and consumables are also fundamental to ensure devices operate efficiently (Castillo et al., 2012; Wood & Mattson, 2014; WHO, 2010). Hence, designers need to simplify the design, for instance, reducing the number of parts may reduce the cost and increase the possibility that parts are available or reproducible in the country (Wood & Mattson, 2014). In our analysis, affordability (n = 13) was the most frequently mentioned technological factor, followed by availability of repair tools, spare and replacement parts (n = 7) and availability of consumables (n = 7). The relevance of these factors was highlighted by interviewees:
Because sometimes there are existing products in terms of the design that might be available for use in developed countries, that might be unaffordable for low-income settings or the materials might be inappropriate or something like that. (I-7; coded as Affordability)
Because a hearing aid battery costs about a dollar and lasts about a week, and obviously it is very expensive a dollar a week in Africa, and also very difficult to find a battery, outside of the capital city. (I-2; coded as Affordability)
Industrial factors describe the ecosystem in which the device will be produced and deployed. Closely linked to the technological factors, these refer to the capacity to manufacture, distribute and commercialize a device. One interviewee commented:
It’s really the lack of distribution and support networks in these countries that makes it so hard [for the device to be distributed]. (I-1; coded as Supply and distribution chains for devices)
Understanding industrial factors provides knowledge of market prices, existing supply chains and availability of consumables and spare parts. The existence of supply and distribution chains for the devices (n = 5) and the quality of the local manufacture (n = 5) were the most frequently mentioned industrial factors, followed by the reliance of the industrial sector on craftsmanship (n = 5) and the existence of competition (n = 3) or commercial monopolies (n = 1).
Physical: Infrastructure, Geographical, and Environmental Factors
The physical context defines the interactions occurring between individuals and objects. The exploration of the physical context is one of the first steps that designers embark on (Langdon, Johnson, Huppert, & Clarkson, 2013; Nemoto et al., 2015). The frameworks by WHO (2010) and Gauthier et al. (2013) refer almost exclusively to these factors, which include infrastructural, geographical and environmental factors.
Infrastructural factors are the most frequently mentioned within this category (n = 58). When infrastructure is poor or not readily available, devices fail to be deployed, distributed and used, ultimately hindering the purpose of the design. Infrastructural factors include the consideration of whether there is electricity or another power supply (n = 17) or whether infrastructure is accessible and available (n = 11), including access to buildings, water systems and transportation. As mentioned by an interviewee:
The first stage is to look at its operating environment. And then look at what the logistical situation is in the area [the device] is going into. Are they going to be able to have access to compressed gases? Or do you have to provide something that will function without them? (I-3; coded as Availability and accessibility of physical infrastructure)
Geographical and Environmental Factors are important when designing medical devices. Environmental conditions can limit options for the use of certain materials and determine how the components of a device operate in a setting. Temperature (n = 10) and humidity (n = 8), as part of the local conditions, are important when considering the operation and lifespan of the devices. One interviewee said:
I’ve been in sort of 28 different African countries with all sorts of environments. It is high humidity. You need someone that is experienced in that sort of environment before they can make decisions on what equipment is suitable. (I-3; coded as Humidity)
Oxygen concentrators, for instance, rely on the conditions of the environment during operation, delivering poor quality of oxygen concentration under high temperature and high humidity conditions (Peel & Howie, 2009). Other important factors include geographical remoteness (n = 7) and roughness of the terrain to access the facility (n = 6).
Individual: Socio-Cultural Factors
Design is a purposeful activity in which the socio-cultural and natural environments are translated into a techno-physical environment (Rosenman & Gero, 1998). User experiences of a product are defined by an individual’s culture, knowledge, attitudes and behaviours (Leblanc, 2009; Nemoto et al., 2015; Stappers et al., 2007). Socio-cultural factors are thus essential to identify genuine problems that designed products need to address (Castillo et al., 2012; Cipolla & Bartholo, 2014; Huang & Deng, 2008; Lin, 2007; Rosenman & Gero, 1998; Stappers et al., 2007).
Socio-cultural factors refer to the characteristics of the users and beneficiaries of the device. Cultural beliefs around health, for instance, are part of the description of the characteristic of the setting in the WHO’s (2010) framework. Designers should place as much attention on socio-cultural aspects as they do on physical and technical aspects (i.e., “look like a real foot”, high-quality finishes). Religious and cultural beliefs (n = 5), literacy level (n = 4), socio-economic stratification (n = 2) and the local language (n = 1) are important considerations. When referring to these factors, an interviewee said:
I think also the family environment, looking at not just the mother but maybe the husband and the kids, and family support in terms of infant health and what their priorities are. I mean, if they only have one day to go to the clinic and it is also the day they need to come into the city to do some shopping, what is more important? (I-10, coded as Religious and socio-cultural beliefs)
Systems and Structures: Political, Institutional, Economic and Public Health Factors
Healthcare rests within a system that comprises organized individuals and institutions who play specific roles in providing care. These organizational systems are forms of collaborative networks of different stakeholders working and communicating for the same purpose, assuring health care is given to the patient (World Health Organization, 2010). They involve individuals, devices, services and infrastructural spaces. The resources available to provide care depend on how the networks are institutionally, politically and economically structured. Being complex themselves, these systems and structures are crucial to facilitate the deployment, adoption and use of medical devices (World Health Organization, 2010). Equally, devices influence the overall performance of the systems (World Health Organization, 2010). If designers overlook these structures, the design might fail (Anderson & Markides, 2007; World Health Organization, 2010). The Systems and Structures category aims to capture these networks (n = 79) and has been divided into Institutional, Economic and Public Health factors.
The institutional factors categorise components of an organization or institution of care. These include knowledge of technology management within the hospital (n = 5), the availability of technical or biomedical technicians (n = 6), facilities and tools for maintenance of devices (n = 3), training for use of the devices (n = 4) and also funding and financing to operate the devices (n = 8). The economic factors refer to aspects such poverty level (n = 6) and a country’s GDP (n = 2). The public health factors describe the systematic determinants of populations’ health. The structure and organization of care provision and public health factors are mutually dependent (e.g., morbidity defining the type of care to be provided). Public health factors include morbidity and mortality indicators (n = 17), awareness of treatments (n = 4), adherence to treatment (n = 1) and communication mechanisms between healthcare providers and patients (n = 1).
A solid knowledge of systems and structures helps address questions of technology deployment, such as supply and distribution systems, and potential business models (Castillo et al., 2012). In most healthcare systems, equipment is purchased through tendering processes based on pricing and generic product descriptions that may not represent genuine needs in the setting. The problems of inadequate specifications and tendering processes (n = 7) were mentioned as barriers for introducing innovative technologies. One interviewee commented:
The government has a subsidies program for wheelchairs […], but it’s only for a wheelchair that is described as a wheelchair by the Indian Institute of Standards. […] So, in the long-term, I think we are going to have to apply and get approval from the Institute of Standards for our wheelchair [because] it’s a different product. That is an issue. (I-4; coded as Appropriateness of specification and tendering processes)
Another interviewee mentioned the challenges of international standards and increase in the prices when purchases are done through tenders:
If you make technical equipment to the international standards, it will not work in that location. […] The standards are a problem. […] Once you get into the international tender arena, to be honest, the costs go through the roof, […] medical equipment bought through tender increased the price 6-fold. (I-3; coded as Appropriateness of specification and tendering processes)
Relevance of Factors by Type of Device
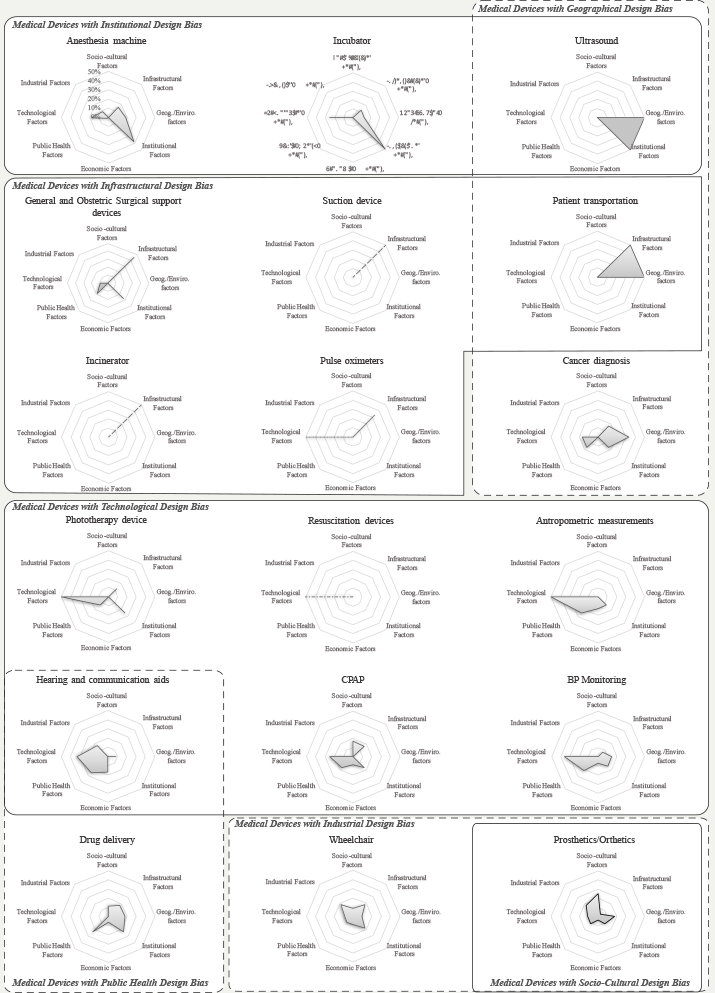
The analysis included the relevance of contextual factors by type of technology. Figure 2 shows some of these differences. For instance, in the case of technologies for personal use (e.g., prosthetic devices), which are often purchased by the user, the Socio-cultural Factors are often cited as highly relevant. Some examples include the need for prosthetic limbs that support religious practices or productive activities such as farming and agriculture. Industrial aspects, especially questions about how to manufacture the technologies, were relevant for wheelchairs and prosthetic devices. For other technologies, such as surgical devices, patient transportation and suction devices, Infrastructural Factors are crucial. Institutional Factors are often more relevant for technologies primarily used in clinical settings than for other technologies. This group includes infant incubators, anaesthetic machines and ultrasounds. Though affordability and cost are often cited as key in frugal healthcare innovations, Economic Factors were not the most frequently mentioned factors for most technologies.
Figure 2. Relevance of contextual factors by the type of device (normalized). (Click the figure to enlarge it.)
To facilitate visualisation, all graphs were fixed to show the percentage of mentions for each category to a maximum of 50% of the total per device. However, for the resuscitation device, suction device and incinerator, the dash-dot line indicates that factors within a single category were mentioned (100%). In the case of the pulse oximeter (dotted line), the technological factors accounted for 66.7% and the infrastructural factors accounted for the rest.
Example of Use
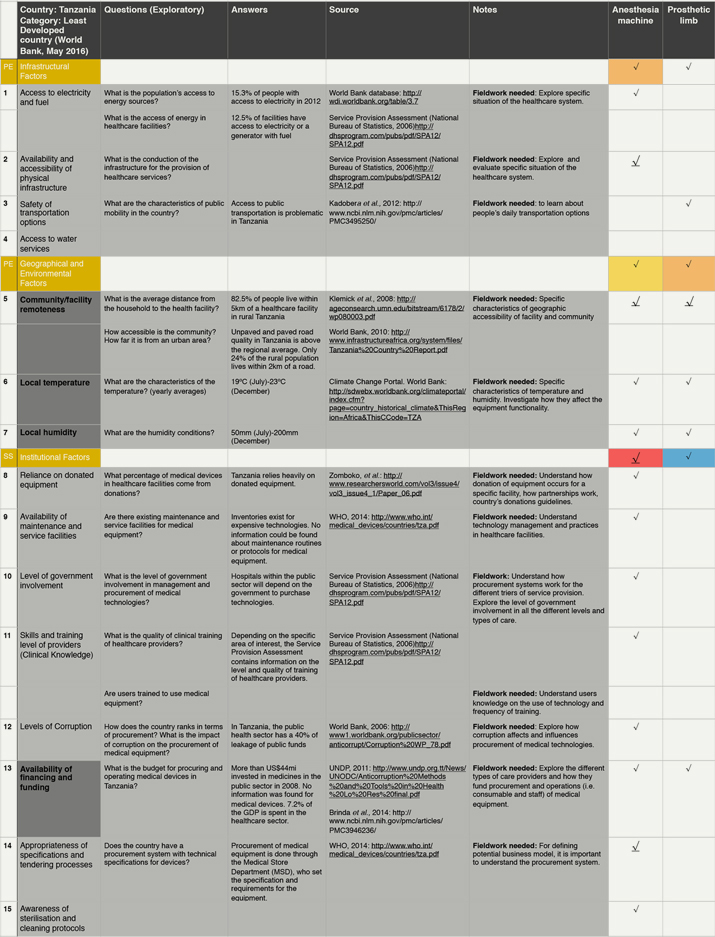
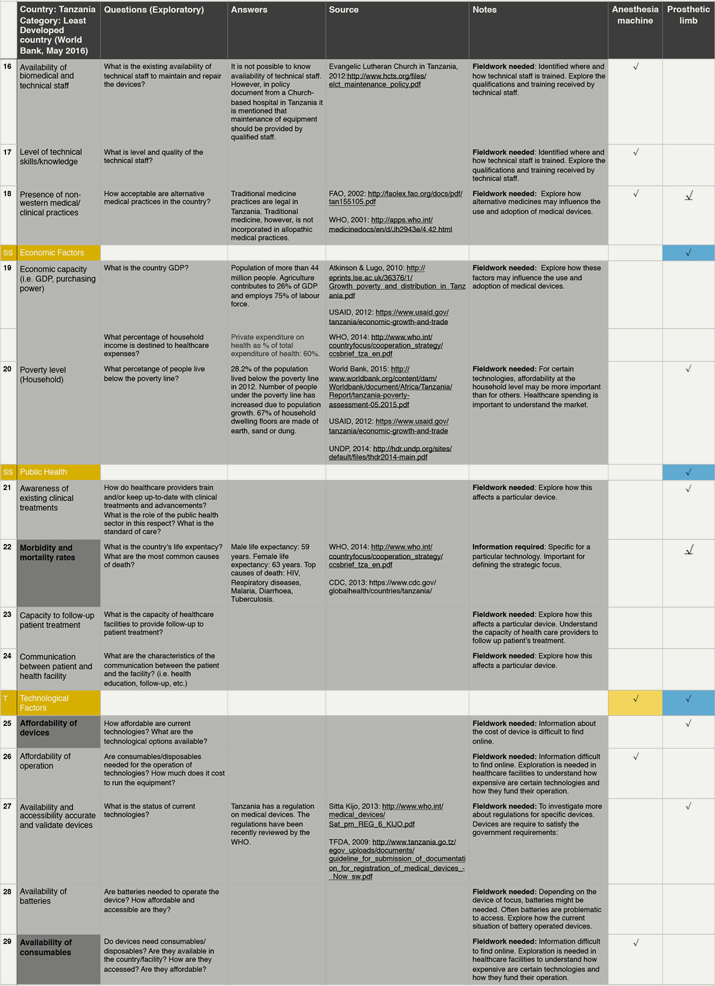
By raising awareness of otherwise underappreciated or even neglected contextual factors, the framework aims to support pre-mortem evaluations of contextually conscious designs and hopefully prevent avoidable failures in the later stages of bringing products into the market. To test the framework in the simplest possible way, we employed it to collect data from an unfamiliar context (Figure 3). In a desktop study on Tanzania, we proposed one or two exploratory questions for each contextual factor in the framework. We then collected data from online sources to help address these questions. Firstly, we visited websites from Government bodies or International Organizations, followed by websites from charitable organizations, academic institutions and news organisations. When data was lacking, we made notes on what needed further exploration through fieldwork for instance.
 |
 |
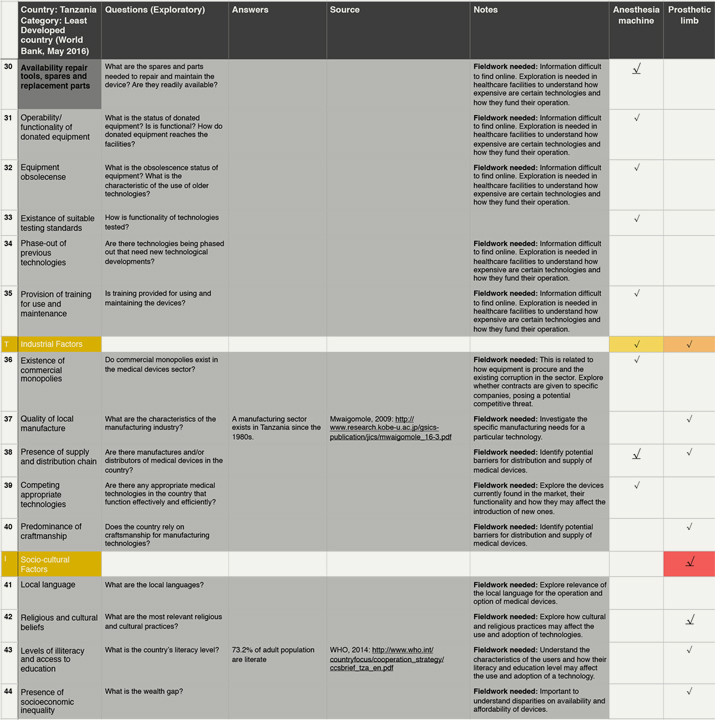 |
Figure 3. Illustrative example of the practical use of the Contextual framework. (Click the figures to enlarge them.)
Test exercise conducted by one researcher to collect information following the contextual factors on the framework. The dark grey cells of the first column highlight the 10 most mentioned factors for all devices. Colours in the cells of the last two columns represent the relevance of the factors for each device: red, highly relevant; orange, relevant; yellow, slightly relevant; blue, little relevance; and white, not relevant. Within each category, the underlined arrows highlight the most mentioned factor from that category.
From the data collected, a preliminary analysis can be drawn to show the implications of the factors on two technologies: anaesthetic machine and prosthetic limb. The most important factors for these technologies are availability and accessibility of physical infrastructure, remoteness of the community and the healthcare facility, appropriateness of the technical specifications and tendering process, availability of spare parts and religious and cultural practices. As can be seen in bold in Figure 3, we focus on these factors for the purpose of this exercise.
Contextual factors influence design in many different ways. For instance, in Tanzania the average temperature and humidity levels in the country are high. Hence, the options for the materials to manufacture a prosthetic limb might be limited by environmental conditions. For the anaesthetic machine, these conditions demand a built-in ventilator to keep the device cool and operational, adding to the energy demands of the system. This is a potential challenge considering that access to electricity in households and healthcare facility is very limited (15.3% and 12.5%, respectively). Moreover, roads do not service most rural populations (only 24% of people live within 2 km of a road). The lack of access to paved roads may affect the distribution and supply of medical gases, spares and even the device itself. The lack of roads is also important for the design of the prosthetic limb. Considering socio-economic factors, most people in Tanzania work in agriculture (75% of labour) and nearly 30% of the population lives below the poverty line. Considering that most people practice a religion, the design of the prosthetic limb should be influenced by these practices (praying) as well as lifestyles (working in agriculture and the need to walk long distances).
Finally, Institutional Factors are relevant for both devices. The country relies heavily on donated equipment and the healthcare sector accounts for a large proportion of funds lost to corruption. Since the majority of purchases for public healthcare facilities are done through the Medical Store Department, devices need to be designed to be able to compete against donated equipment and also perhaps for the implementation of business models that can help tackle corruption.
The example shows how to use the framework as a taxonomical tool for data collection for problem scoping. Critically, it demonstrates how the information ascertained using the tool can have a real influence on the design itself, from material selection to functional characteristics. Deep knowledge of the context can only be gained through contextual immersion, but an initial extensive scoping exercise can be conducted with these contextual tools to gain a basic understanding of context during the ideation process.
Discussion
The year 2015 marked the end of the Millennium Development Goals. Unfortunately, global health equality remains a goal far from being achieved. Technologies and infrastructure are crucial for healthcare provision and to achieve universal healthcare coverage we will require appropriate technological advances. To support the most vulnerable and marginalized people of the world, the design of medical devices needs to account for the varied contexts in LRSs and to recognise their unique challenges from the earliest stages of design. In this paper, we present the development of a holistic contextual framework for medical devices in LRSs to assist in this process.
Recognising the Social and Organisational Aspects of Design
The challenge of the lack of access to medical devices in LRSs is a multifaceted problem that requires an understanding that goes beyond the mere characteristics of the users’ interactions with the technologies. Designing medical devices for LRSs must also go further than designing for the basic concepts of frugality, simplicity, low-cost and scarcity. Although these elements are crucial for devices to reach healthcare facilities in LRSs, ranking highly in the framework, the design should also address a broader scope. This scope should include the complexity of the context and place technologies within dynamic contexts that set a problem in socio-technical systems (Bijl-Brouwer & Voort, 2014).
Unfortunately, the complexity of challenges in designing devices for LRSs tend to be poorly understood and is often overlooked (Hall, Matos, & Martin, 2014). In our research, we found that the practice of designing medical devices for LRSs is currently a highly technically-oriented task. Although the Structures and Systems category had the highest ranking individual factor (Morbidity and mortality rates), the Physical and Technical categories contained the majority of the other top ranking factors. Of the ten most frequently mentioned factors, five belong to the Physical category—energy, infrastructure and environmental conditions being the most frequently mentioned—and three to the Technical category. Many of these factors may be considered well understood, however, our approach highlights the relevance of less considered contextual factors, some of which may ultimately prove to be critical determinants of use.
When designing for LRSs, an approach inclusive of systems and organizations will encourage innovation processes that enable access to products and services for those most in need (Castillo et al., 2012). In areas such as design for sustainability, frameworks have successfully assisted designers to identify design solutions to problems in LRSs that are inclusive of the socio-cultural dimensions. Similarly, the present framework accounts for a broader range of contextual factors and places the technology within an organizational system in which healthcare is delivered. By looking at the context as a socio-technical system, designs may be able to achieve technological possibilities that will not only be technically-sound or frugal, but will also be adopted in communities by satisfying people’s tacit and latent needs (Stappers, van Rijn, Kistemaker, Hennink, & Sleeswijk Visser, 2009). In practice, it is likely that more than one solution exists to solve these healthcare challenges, yet a purely technical approach is likely to fail to identify all possibilities (Bijl-Brouwer & Voort, 2014).
Contextual Framework as A Tool for Designers
A deep contextual understanding is required for designing, developing and commercializing products in LRSs (Castillo et al., 2012; Jagtap, Larsson, & Kandachar, 2013a). However, the identification of requirements and decision-making in the design process are generally unreported in the studies we have identified. Moreover, there is a lack of design-related tools to support these processes.
Frameworks, tools and methods to support designers in the initial phases of the design process are fundamental to better identify the needs for a product and understand its context of use (Chakrabarti, Morgenstern, & Knaab, 2004). Our framework aims to serve this purpose by providing a sound taxonomic tool to support designers to understand the context of use of medical devices in LRSs. The framework is not intended to be prescriptive and, as such, designers need to discern how information about these factors will affect the design of a device. As shown in the use example, the framework could be useful for the identification of needs and scoping of the problem at the early stages of the design process. The data collected by designers using this taxonomic framework could serve to inform, build and update the design of a product during later stages of the design process (e.g., validating and verifying design requirements) and may help to make the design decisions more explicit and transparent.
Conclusion
This research has used academic design cases and perspectives from experts in the field to explore the understanding of the context of use for medical devices in resource limited settings. Through systematically reviewing literature and conducting a qualitative analysis of data, the research resulted in a framework that we hope will help designers to inform their initial contextual explorations. We believe that the exercise of explicitly describing the identification and selection of factors is open to critique, but it is also an activity that should be encouraged by design researchers and practitioners. We hope that this research motivates similar research in the field. From the study, the main conclusions that can be drawn are:
- Contextual information is critical for problem understanding during the process of designing products for LRSs, particularly in the case of medical devices. Understanding and studying the context, however, poses several challenges for designers unfamiliar with this type of settings or with little experience designing. Not only do designers need to challenge their own assumptions and worldviews, but the task itself is costly and time-consuming.
- The framework in the study offers a taxonomical tool to support designers in the collection of data, thus making the initial exploration more efficient and effective. The framework provides guidance rather than prescriptive rules. The relevance of the factors varies depending on the type of technology being designed, but the context itself will determine the relevance of the factors (see, for instance, the example of use). The framework, however, does not aim to substitute contextual immersion, but rather to help designers during ideation and throughout the design process.
- As shown in the framework, the context of a technology goes beyond mere technical elements. Ultimately, whether a technology is adopted and diffused depends on the elements of the wider system that embraces technologies and their users. In this sense, we would like to highlight the relevance of the ‘Systems and Structures’ factors as elements that need to be considered by designers when designing medical devices for LRSs. These elements may be critical to whether a device will be purchased and used in healthcare settings, for example, organisational resources, procurement mechanisms, customs and traditions.
- Design researchers need to encourage more explicit ways to document the identification and selection of contextual factors. The study shows that academic publications offer a rather general description of the context, as opposed to the designer’s approach to the description, this being often enriched by their own experience in the context and development of the product. This study has presented a comprehensive description of the decision-making process to build the framework in the hope of encouraging other researchers to do likewise. Learning from the methods used by other researchers in design will assist us to improve design practice to tackle the world’s most critical challenges.
Implications in Research and Practice
Designers face challenges when designing medical devices for LRSs. In this regard, the framework may prove useful in engineering and industrial design education. Using the framework, students may start ideating by learning and becoming aware of the nature of problems faced in LRSs. The supplementary example of use in Appendix shows that understanding the context could influence the technical side of the design in multiple ways, for instance, how environment influences the selection of the materials or how road accessibility may influence the design of parts. We consider that the approach presented can help deliver more effective designs of medical devices for LRSs, with a greater chance of crossing the “last mile” of the product development process. This broader scope may thus offer new perspectives to tackle global health challenges.
Our contextual framework, based on the systematic literature review and empirical research, could be used by designers to collect contextual information. In design research, we believe this study will help researchers to rethink and question our conceptions and pre-conceptions of unfamiliar contexts. Although we are aware that this framework is one proposal to improve design success, we would like to invite designers and researchers to use it, adapt it and improve it with their own experiences and research in the field.
Limitations and Further Research
In its current state, the framework has been developed specifically for medical devices, one limitation being the focus on healthcare-related environments. Although the categorical factors may be applicable to other products, further research is required to explore the applicability of the framework to other product areas in LRSs. We believe that some minor adaptation to the framework may be required for that purpose.
We also acknowledge the lack of guidance on when and how to best use the framework at the different stages of the process as a limitation. Further research is needed to test the framework as a practical design support. Research may help to understand the usefulness of the framework in the design process and even understand the relevance of the categories at different stages of the process (i.e., is it useful to learn about a category in the early- or late-design stages?). We hope that this framework encourages similar approaches that can provide a starting point for an integrative approach towards designing effective and functional products for LRSs.
Acknowledgments
The National Council of Science and Technology (CONACYT) in Mexico supported this research.
References
- Advancing Safety in Healthcare Technology. (2015). ANSI/AAMI/IEC 62366-1: 2015. Arlington, VA: Association for the Advancement of Medical Instrumentation.
- Alexander, K., & Clarkson, J. (2000a). Good design practice for medical devices and equipment, Part I: A review of current literature. Journal of Medical Engineering & Technology, 24(1), 5-13.
- Alexander, K., & Clarkson, J. (2000b). Good design practice for medical devices and equipment, part II: Design for validation. Journal of Medical Engineering & Technology, 24(2), 53-62.
- Alexander, K., & Clarkson, J. (2002). A validation model for the medical devices industry. Journal of Engineering Design, 13(3), 197-204.
- Anderson, J., & Markides, C. (2007). Strategic innovation at the base of the pyramid. MIT Sloan Management Review, 49(1), 83-88.
- Arasaratnam, A., & Humphreys, G. (2013). Emerging economies drive frugal innovation. Bulletin of the World Health Organization, 91(1), 6-7.
- Bergmann, J. H. M., Noble, A., & Thompson, M. (2015). Why is designing for developing countries more challenging? Procedia Manufacturing, 3, 5693-5698.
- Bezuidenhout, H., Woods, D., Wyatt, J., Lawn, J. (2006). Does fetal heart rate count? Developing a low cost, alternative powered doppler fetal heart monitor for use in low resource high mortality settings. In Proceedings of the 4th IET Seminar on Appropriate Healthcare Technologies for Developing Countries (pp. 155-161). London, UK: IET. doi: 10.1049/ic.2006.0673
- Bijl-Brouwer, M., & Voort, M. C. (2014). Understanding design for dynamic and diverse use situations. International Journal of Design, 8(2), 29-42.
- Braun, R., Benedict, M., Wendler, H., & Esswein, W. (2015). Proposal for requirements driven design science research. In B. Donnellam, M. Helfert, J. Kenneally, D. VanderMeer, M. Rothenberger, & R. Winter (Eds.), New horizons in design science: Broadening the research agenda (Vol. 9073, pp. 135-151). Cham, Switzerland: Springer International Publishing.
- Castillo, L. G., Diehl, J. C., & Brezet, J. C. (2012). Design considerations for base of the pyramid (BoP) projects. Retrieved November 8, 2016, from http://docplayer.net/12473633-Design-considerations-for-base-of-the-pyramid-bop-projects.html
- Chakrabarti, A., Morgenstern, S., & Knaab, H. (2004). Identification and application of requirements and their impact on the design process: A protocol study. Research in Engineering Design, 15(1), 22-39.
- Chaturvedi, J., Logan, A., Narayan, G., & Kuttappa, S. (2015). A structured process for unmet clinical need analysis for medical device innovation in India: Early experiences. BMJ Innov. doi:10.1136/bmjinnov-2014-000010
- Cipolla, C., & Bartholo, R. (2014). Empathy or inclusion: A dialogical approach to socially responsible design. International Journal of Design, 8(2), 87-100.
- Donaldson, K. M. (2006). Product design in less industrialized economies: Constraints and opportunities in Kenya. Research in Engineering Design, 17(3), 135-155.
- Edwards, M. J. (2008). Designing a digital anthropometric measurement system for hospitals in Malawi. In Proceedings of the 5th IET Seminar on Appropriate Healthcare Technologies for Developing Countries, (pp. 1-6). London, UK: IET.
- Free, M. (2004). Achieving appropriate design and widespread use of health care technologies in the developing world. International Journal of Gynaecology and Obstetrics, 1(85), S3-13.
- Garrett, L. (2007). The challenge of global health. Foreign Affairs, 86(1), 14-38.
- Gauthier, A. K., Cruz, G., Medina, L., & Duke, S. (2013). Design factors for medical device functionality in developing countries. In Proceedings of the Annual Conference of the Institute of Industrial Engineers. Retrieved November 8, 2016, from https://www.highbeam.com/doc/1P3-3169589351.html
- Green, M. G. (2005). Enabling design in frontier contexts: A contextual needs assessment method with humanitarian applications. Retrieved November 8, 2016, from https://repositories.lib.utexas.edu/bitstream/handle/2152/2431/greend58907.pdf?sequence=2&isAllowed=y
- Green, M. G., Linsey, J. S., Seepersad, C. C., Wood, K., & Jensen, D. (2006). Frontier design: A product usage context method. Retrieved November 8, 2016, from https://www.me.utexas.edu/~ppmdlab/files/PUC%20ASME%20DETC%20DTM%20MG%202006%20v1.0%20DRAFT%20Submitted.pdf
- Hall, J., Matos, S. V., & Martin, M. J. C. (2014). Innovation pathways at the base of the pyramid: Establishing technological legitimacy through social attributes. Technovation, 34(5-6), 284-294.
- Howitt, P., Darzi, A., Yang, G. -Z., Ashrafian, H., Atun, R., Barlow, J., …Wilson, E. (2012). Technologies for global health. The Lancet, 380(9840), 507-535.
- Huang, K. H., & Deng, Y. S. (2008). Social interaction design in cultural context: A case study of a traditional social activity. International Journal of Design, 2(2), 81-96.
- Jagtap, S., Larsson, A., & Kandachar, P. (2013a). Design and development of products and services at the base of the pyramid: A review of issues and solutions. International Journal of Sustainable Society, 5(3), 207-231.
- Jagtap, S., Larsson, A., Hiort, V., Olander, E., Warell, A., & Khadilkar, P. (2014). How design process for the base of the pyramid differs from that for the top of the pyramid. Design Studies, 35(5), 527-558.
- Jagtap, S., Larsson, A., Hjort af Ornäs, V., Olander, E., & Warell, A. (2013b). Fighting poverty through design: Comparing design processes for the base and the top income pyramid. In Proceedings of the 19th International Conference on Engineering Design (pp. 19-22). Seoul, Korea: Sungkyunkwan University.
- Langdon, P., Johnson, D., Huppert, F., & Clarkson, J. (2013). A framework for collecting inclusive design data for the UK population. Applied Ergonomics, 46, Part B, 318-324.
- Lawrence, C. (2014). Why the next generation of designers will save the world. Design Management Review, 25(2), 42-46.
- Le Cocq, A. D. (1987). Application of human factors engineering in medical product design. Journal of Clinical Engineering, 12(4), 271-278.
- Leblanc, T. (2009). Design vs. re-design, and how to innovate. In Proceedings of the 11th International Conference on Engineering and Product Design Education (pp. 348-353), Brighton, UK: The Design Society.
- Lin, R. T. (2007). Transforming Taiwan aboriginal cultural features into modern product design: A case study of a cross-cultural product design model. International Journal of Design, 1(2), 45-53.
- Hekkert, P., & van Dijk, M. (2011). Vision in product design [ViP]:A guide for innovators. Amsterdam, the Netherlands: BIS.
- Maguire, M. (2001). Methods to support human-centred design. International Journal of Human-Computer Studies, 55(4), 587-634.
- Malkin, R. A. (2007a). Barriers for medical devices for the developing world. Expert Review of Medical Devices, 4(6), 759-763.
- Malkin, R. A. (2007b). Design of health care technologies for the developing world. Annual Review of Biomedical Engineering, 9, 567-587.
- Malkin, R., & Anand, V. (2010). A novel phototherapy device: The design community approach for the developing world. IEEE Engineering in Medicine and Biology Magazine, 29(2), 37-43.
- Manzini, E. (2014). Making things happen: Social innovation and design. Design Issues, 30(1), 57-66.
- Manzini, E., & Coad, R. (2015). Design, when everybody designs: An introduction to design for social innovation. Cambridge, MA: MIT Press.
- Martin, J. L., Clark, D. J., Morgan, S. P., Crowe, J. A., & Murphy, E. (2012). A user-centred approach to requirements elicitation in medical device development: A case study from an industry perspective. Applied Ergonomics, 43(1), 184-190.
- Medina, L. A., Wysk, R. A., & Okudan Kremer, G. E. (2011). A review of design for X methods for medical devices: The introduction of a design for FDA approach. In Proceedings of the 16th Conference on Design for Manufacturing and the Life Cycle (pp. 849-861). New York, NY: ASME.
- Mohedas, I., Daly, S. R., & Sienko, K. H. (2014a). Design ethnography in capstone design: Investigating student use and perceptions. International Journal of Engineering Education, 30(4), 888-900.
- Mohedas, I., Daly, S. R. & Sienko, K. H. (2014b). Gathering and synthesizing information during the development of user requirements and engineering specifications. In Proceedings of the ASEE Annual Conference & Exposition. Indianapolis, IN: ASEE.
- Mohedas, I., Daly, S. R., & Sienko, K. H. (2015). Requirements development: Approaches and behaviors of novice designers. Journal of Mechanical Design, 137(7), 1-10.
- Moultrie, J., Sutcliffe, L., & Maier, A. (2015). Exploratory study of the state of environmentally conscious design in the medical device industry. Journal of Cleaner Production, 108, part A, 363-376.
- Mulgan, G., Tucker, S., Ali, R., & Sanders, B. (2007). Social innovation: What it is, why it matters and how it can be accelerated. London, UK: The Young Foundation.
- Nakata, C., & Weidner, K. (2012). Enhancing new product adoption at the base of the pyramid: A contextualized model. Journal of Product Innovation Management, 29(1), 21-32.
- Nemoto, Y., Uei, K., Sato, K., & Shimomura, Y. (2015). A context-based requirements analysis method for PSS design. Procedia CIRP, 30, 42-47.
- Niemeier, D., Gombachika, H., & Richards-Kortum, R. (2014). How to transform the practice of engineering to meet global health needs. Science, 345(6202), 1287-1290.
- Nimunkar, A. J., Baran, J., Van Sickle, D., Pagidimarry, N. K. & Webster, J. G. (2009). Medical devices for developing countries: design constraints and approaches. In Proceedings of the Conference on Medicine and Biology Society (pp. 7048-7051). Minneapolis, MN: IEEE.
- Peel, D., & Howie, S. R. C. (2009). Oxygen concentrators for use in tropical countries. Journal of Clinical Engineering, 34(4), 205-209.
- Roblyer, D. et al., 2007. Objective screening for cervical cancer in developing nations: Lessons from Nigeria. Gynecologic Oncology, 107 (1 SUPPL.), S94-97.
- Rodriguez, J., Diehl, J. C., & Christiaans, H. (2006). Gaining insight into unfamiliar contexts: A design toolbox as input for using role-play techniques. Interacting with Computers, 18(5), 956-976.
- Rosenman, M. A., & Gero, J. S. (1998). Purpose and function in design: From the socio-cultural to the techno-physical. Design Studies, 19(2), 161-186.
- Roser, H. M., & Walker, P. D. (2014). Managing the conceptualization process in innovative engineering design, demonstrated on a hybrid two-wheeler design case study. In Proceeding of the 12th Conference on Engineering Systems Design and Analysis (pp. 1-10). New York, NY: ASME.
- Saldana, J. (2013). The coding manual for qualitative researchers. London, UK: Sage.
- Schifferstein, H., & Hekkert, P. (2008). Product experience. San Diego, CA: Elsevier.
- Sharp, M. (1994). The jaipur limb and foot. Medicine and War, 10(3), 207-211.
- Sienko, K. H., Sarvestani, A. S., & Grafman, L. (2013). Medical device compendium for the developing world: A new approach in project and service-based learning for engineering graduate students. Global Journal of Engineering Education, 15(1), 13-20.
- Sinha, S. R., & Barry, M. (2011). Health technologies and innovation in the global health arena. New England Journal of Medicine, 365(9), 779-782.
- Soegaard, M., & Dam, R. F. (n.d.). Context of use. Retrieved November 8, 2016, from https://www.interaction-design.org/literature/book/the-glossary-of-human-computer-interaction/context-of-use
- Stappers, P. J., Hekkert, P., & Keyson, D. (2007). Design for interaction: Consolidating the user-center design focus in industrial design engineering. In Proceedings of the Conference on Engineering and Product Design Education. Newcastle Upon Tyne, UK: Design Society.
- Stappers, P. J., van Rijn, H., Kistemaker, S. C., Hennink, A. E., & Sleeswijk Visser, F. (2009). Designing for other people’s strengths and motivations: Three cases using context, visions, and experiential prototypes. Advanced Engineering Informatics, 23(2), 174-183.
- The World Bank. (2015). Poverty overview. Washington, DC: The World Bank.
- Waddell, S. (2012). Design guidelines to address global challenges: Lessons from global action networks. Journal of Organization Design, 1(3), 1-19.
- Wood, A. E., & Mattson, C. A. (2014). A method for determining customer needs in the developing world. In Proceedings of the 40th Conference on Design Automation (No. V02AT03A047). New York, NY: ASME.
- Wood, A. E., & Mattson, C. A. (2016). Design for the developing world: Common pitfalls and how to avoid them. Journal of Mechanical Design, 138(3), 1-11.
- World Health Organization. (2010). Context dependency of medical devices. Retrieved November 8, 2016, from http://whqlibdoc.who.int/hq/2010/WHO_HSS_EHT_DIM_10.5_eng.pdf
Appendix
Appendix 1. Decision tree for inclusion of studies (Aranda-Jan, Cruickshank, & Moultrie, 2015).
Appendix 2. Decision tree for inclusion of contextual factors.
Appendix 3. Holistic Framework (Ranked Factors).
Appendix 4. Technical Factors (Industrial and Technological). Numbers refer to the number of times each contextual factor was mentioned.
Appendix 5. Infrastructural Factors. Numbers refer to the number of times each contextual factor was mentioned.
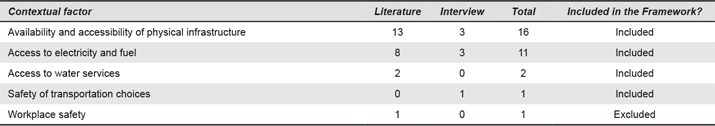
Appendix 6. Geographical and Environmental Factors. Numbers refer to the number of times each contextual factor was mentioned.
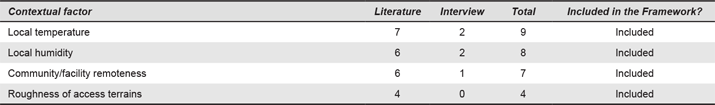
Appendix 7. Socio-cultural Factors. Numbers refer to the number of times each contextual factor was mentioned.
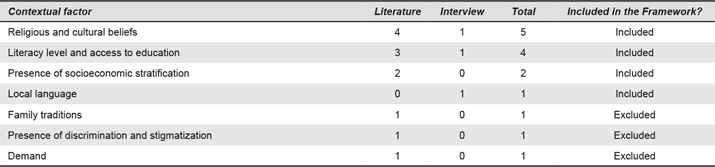
Appendix 8. Structures and Systems Factors. Numbers refer to the number of times each contextual factor was mentioned.