Play Experiences for People with Alzheimer’s Disease
Hester Anderiesen 1,*, Erik Scherder 2,3, Richard Goossens 1,4, Valentijn Visch 1,
and Laura Eggermont 2
1 Delft University of Technology, the Netherlands
2 VU University Amsterdam, the Netherlands
3 University of Groningen, the Netherlands
4 Erasmus Rotterdam, the Netherlands
Little is known about the experience of people with dementia while playing games. This might be a reason why hardly any games are specifically designed for this group. We aimed to determine which play experiences can be expected to be suitable for persons in different stages of Alzheimer’s disease (AD). Twenty-two play experiences were related to the neuropathology that is characteristic of the different stages of dementia: earliest, mild-to-moderate, and severe. This literature overview is based on neuroimaging, neuropathological, and clinical studies. We found that for all older persons with AD, regardless of disease severity, the play experiences sensation, relaxation, and reminiscence are likely to be suitable. The play experiences nurture, sympathy, fellowship, expression, humour, eroticism, subversion, and challenge may be appropriate only for those in the earliest and mild-to-moderate stages of AD. The play experience exploration is most likely not suitable, irrespective of the stage of AD. For the remaining play experiences we did not find sufficient evidence to draw conclusions. We conclude that the choice of play experiences in game design for older persons with AD is dependent on disease stage. Current recommendations may contribute to tailor-made games that are suitable for different persons with AD.
Keywords – Alzheimer’s Disease, Dementia, Game Design, Play Experiences, Playful User–Product Interactions
Relevance to Design Practice – This study informs designers of games and of playful user–product interactions about which play experiences are suitable for older persons in the different stages of Alzheimer’s disease.
Citation: Anderiesen, H., Scherder, E., Goossens, G., Visch, V., & Eggermont, L. (2015). Play experiences for people with Alzheimer’s disease. International Journal of Design, 9(2), 155-165.
Received April 8, 2014; Accepted May 25, 2015; Published August 31, 2015.
Copyright: © 2015 Anderiesen, Scherder, Goossens, Visch, & Eggermont. Copyright for this article is retained by the authors, with first publication rights granted to the International Journal of Design. All journal content, except where otherwise noted, is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 2.5 License. By virtue of their appearance in this open-access journal, articles are free to use, with proper attribution, in educational and other non-commercial settings.
*Corresponding Author: hester@activecues.com.
Hester Anderiesen is a PhD candidate in the Faculty of Industrial Design Engineering at Delft University of Technology. During her PhD research she developed games for older people with dementia. Building on this work, she recently founded a start-up company, Active Cues BV, to supply her games to nursing homes.
Erik Scherder is professor and head of the Department of Clinical Neuropsychology in the Faculty of Psychology and Education at the VU University of Amsterdam, and professor in the Faculty of Behavioral and Social Sciences and the Faculty of Medical Sciences at the University of Groningen.
Richard Goossens is professor of Physical Ergonomics and Coordinator Healthcare Program within the section Applied Ergonomics and Design of the Faculty of Industrial Design Engineering at Delft University of Technology, and professor of Physical Ergonomics in the Department of Neuroscience at Erasmus MC in Rotterdam.
Valentijn Visch is assistant professor in the section Design Aesthetics of the Faculty of Industrial Design Engineering at Delft University of Technology.
Laura Eggermont was assistant professor in the Department of Clinical Neuropsychology in the Faculty of Psychology and Education at the VU University of Amsterdam at the time of this research. She currently works as team leader Clinical Studies Neuro at Nutricia Research Utrecht.
Introduction
The interest of older persons in playing games is shown by various studies from the mid-1970s and 1980s (for a review: Whitcomb, 1990). Although the actual number of gamers over 65 years of age is unknown (Ijsselsteijn, Nap, de Kort, & Poels, 2007), the group is growing as our society is aging (Sharkey & Sharkey, 2011). This aging of society also results in a growing segment of older persons with dementia (Qiu, de Ronchi, & Fratiglioni, 2007), most of whom have Alzheimer’s disease (AD) (Brunnström, Gustafson, Passant, & Englund, 2009). The body of evidence is growing for the therapeutic value of playing games for older persons (Griffith, 2005). Playing games can for example slow down the deterioration of, or might even improve, memory, hand-eye coordination, reaction times, and self-esteem (for an overview: Griffith, 2005). Also, people with AD often have a sedentary lifestyle (Scherder, Bogen, Eggermont, Hamers, & Swaab, 2010), and we argue that playing games can be an opportunity to stimulate them to more physical and social activity. Unfortunately, knowledge on how to design games that match the capacities of this target group is still in its infancy.
The majority of commercial games involve fast interactions, high tones, and small typography (Whitcomb, 1990), and rarely meet the interests of older gamers (Ijsselsteijn et al., 2007). However, the number of studies concerning game design for elderly people is growing rapidly and, although at a less mature level, also for people with dementia. McCallum and Boletsis (2013) reviewed serious games for dementia, and reported mostly positive health effects and successful implementation. However, the majority of studies only included persons with mild cognitive impairment (MCI) and mild AD, whereas no studies reported on people with moderate to severe dementia. Elderly care professionals acknowledge the potential of serious games in the care of people with dementia, but emphasize the importance of designing them specifically for this group (Robert et al., 2014). Bouchard, Imbeault, Bouzouane, and Menelas (2012) addressed the fact that most of the existing games were not suitable for the perceptual and interaction needs of persons with AD, especially in the more advanced stages of the disease, and they presented specific guidelines for designing serious games for people with dementia. Fua, Gupta, Pautler, and Farber (2013) assessed gameplay mechanics, Ijsselsteijn et al. (2007) evaluated digital game interfaces as to their suitability for cognitively impaired older gamers, and Cherniack (2011) and Lewis and Rosie (2012) reviewed literature considering the application of virtual reality in games and rehabilitation for older persons with cognitive disorders. In addition to the existing literature, the present literature overview focuses on the experience of persons with AD when playing games, rather then game design elements, and includes all severities of the disease. Moreover, the play experiences discussed in this paper are not restricted to digital or video games, but also include tabletop games, outdoor games, and serious games. Therefore, we use the term game for all of these subtypes of games.
A person’s cognitive, behavioural, and emotional functioning is closely related to the pathology in the brain (neuropathology) that is characteristic of AD (Samanta, Wilson, Santhi, Kumar, & Suresh, 2006), and may also affect the experiences of persons with AD while playing games. Moreover, their ability to experience play may change during the course of the disease. A wide array of play experiences can be found in literature, such as competition, exploration, fellowship, challenge, thrill, and so forth (Korhonen, Montola, & Arrasvuori, 2009). As far as the authors know, there is no literature describing which play experiences may still be experienced by people with AD over the course of the disease (see Figure 1).
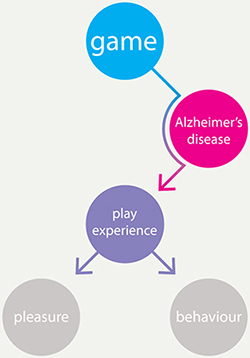
Figure 1. The play experience of games by people with Alzheimer’s disease.
In this paper, we address the question of which play experiences can be expected to be suitable for persons in different stages of AD. We used neuroimaging, neuropathological, and clinical studies to review play experiences of persons with AD. Answering this question is important for several reasons: Firstly, play experiences that rely too much on brain structures that are severely affected by the disease could be experienced by the person with AD to be meaningless and may lead to frustration (Lucero, Kijek, Malone, Santos, & Hendrix, 2000). Secondly, play experiences that are cognitively challenging for the person with AD may be more beneficial from a healthcare perspective than play experiences that are not challenging (Fratiglioni, Paillard-Borg, & Winblad, 2004). Thirdly, play experiences that are cognitively challenging will be most enjoyable (Flores et al., 2008). Finally, appropriate leisure products for persons with AD are scarce, but may enhance their quality of life and may support caregivers in providing good-quality care (Lucero et al., 2000).
We will first provide background knowledge from the game design field, followed by relevant insights from the neuropathology of AD. Subsequently, we will review 22 play experiences, 21 as defined by Korhonen et al. (2009) and an additional one proposed by ourselves, on their appropriateness for older persons in the different stages of AD.
Knowledge from Both Worlds
Games and Play
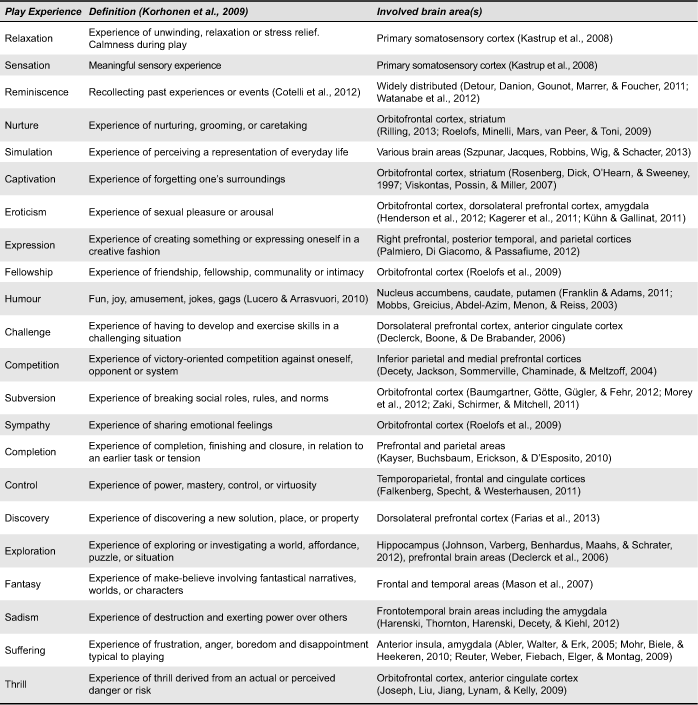
The historian Johan Huizinga (1938/1955) influenced thinking about the value of play with his classic definition: “a free activity standing quite consciously outside ‘ordinary’ life as being ‘not serious’ but at the same time absorbing the player intensely and utterly” (p. 13). Although we tend to associate the absorbing experience of play with children running around the schoolyard, we play throughout our entire lifespan. Caillois (1958/2001) defined two different forms of play: “ludus,” referring to a “game” entailing rule-structured playing and often including competitive behaviour; and “paidia,” representing “play” as a more free-form and improvisational playful behaviour. Whether play is more ludus- or more paidia-like depends on the game design, but even more on the player’s behaviour and experience: a ludus-like chess game can be played exploratively and a paidia dance party can be played competitively. Korhonen et al. (2009) systemically collected and defined all play experiences between ludus and paidia. Their categorization of 21 playful experiences identifies: captivation, challenge, competition, completion, control, discovery, eroticism, exploration, expression, fantasy, fellowship, humour, nurture, relaxation, sadism, sensation, simulation, subversion, suffering, sympathy, and thrill (see Table 1 for definitions). In addition, we propose a complementary play experience, especially for the older generation: reminiscence. In literature, games for older people often appeal to the sentiment of the past and elicit a reminiscing experience, as for example the game “The Chitchatters” (Van Rijn, van Hoof, & Stappers, 2010). Reminiscence, i.e., the act or process of recollecting past experiences or events (Cotelli, Manenti, & Zanetti, 2012) can be compared to fantasy, but in a different computation of time. To understand which of these experiences can be potentially elicited in people with AD we should take the AD neuropathology into account.
Table 1. Play experiences as defined by Korhonen et al. (2009) and involved brain areas.
AD Neuropathology
A detailed description of the neuropathology of AD is beyond the scope of this review. At a more global level, the neuropathology of AD includes, among other things, amyloid plaques (accumulation of amyloid, a protein, between neurons), neurofibrillary tangles (twisted masses of protein fibers within neurons), and atrophy (shrinkage of neurons) (Nelson et al., 2011), leading to a marked decrease in neuronal activity (Kern & Behl, 2009). In AD, shrinkage of neurons and lesions of the pathways connecting different brain areas (i.e., white matter lesions) increase during the disease process (Kester & Scheltens, 2009). Although the speed of the process and the clinical symptoms may vary between persons, the distinct AD neuropathology follows a similar course (Braak & Braak, 1991; Villemagne & Rowe, 2013). In the earliest stage of AD, neuropathology develops in the medial temporal lobe, particularly in the hippocampus (Braak & Braak, 1991; Bastos Leite, Scheltens, & Barkhof, 2004; Ewers et al., 2011). This neuropathology, further developing throughout the mild-to-moderate stage, disrupts important networks connecting the medial temporal lobe and the frontal lobe (Bastos Leite et al., 2004; Scherder, Eggermont, Visscher, Scheltens, & Swaab, 2011), and additional cortical thinning of the temporal and posterior parietal lobes is seen (Ewers et al., 2011). In the most advanced stage, according to both post-mortem research (Braak & Braak, 1991; Swaab, Dubelaar, Scherder, van Someren, & Verwer, 2003) and neuroimaging studies (Ewers et al., 2011), the primary somatosensory cortex shows least AD-related degeneration.
Textbox 1: Consequences of Alzheimer’s disease in daily life Earliest stage: A person has difficulty concentrating, has a decreased memory of recent events, and experiences difficulties in financial management or in travelling alone to new locations. This interferes with daily activities. Socialization may become difficult and the person may therefore start to withdraw from family or friends. Mild-to-moderate stage: A person in this stage has more profound memory deficiencies. Assistance will be needed to complete daily activities such as dressing oneself. Memory loss may include major relevant aspects of current life, such as confusion about time and location. The person will lack good judgment, will have great difficulty handling problems, and will have few interests. Advanced stage: A person in the advanced stage of AD requires extensive assistance during daily activities. They cannot take part in community affairs outside the home and close family members may not be recognized. The person may remember only some details of earlier life. Incontinence, personality changes, delusions, repetitive behaviours such as wandering, and agitation may occur. Based on the Global Deterioration Scale (Reisberg, Ferris, de Leon, & Crook, 1982) and Clinical Dementia Rating (Morris, 1993). |
Merging Knowledge from Both Worlds
In our recommendation as to whether to facilitate certain play experiences in games for persons with AD we used the following requirements: It is appropriate to design for play experiences in games when the brain areas required for these experiences: 1) are still relatively intact; and 2) are slightly affected, but may still be able to respond to an external stimulus. Due to the progressive course of AD, brain areas become more and more affected (Braak & Braak, 1991; Scherder, Eggermont, Visscher, Scheltens, & Swaab, 2011). We included neuroimaging studies (studies that use scanning instruments to interpret damage to and remaining activity of the brain) and neuropathological studies (studies that examine brains post mortem) to review which brain areas relevant for the 22 play experiences are still relatively intact in the different stages of the disease. However, it is difficult to conclude from these studies when there is a change from “experiences being affected” to “an inability to experience.” We therefore also included clinical studies (studies that evaluate those behaviours of persons with AD that reflect certain play experiences, for instance studies that report the presence of a play experience in persons with AD, such as humour). In view of the different neuropathological stages of AD (Braak & Braak, 1991), we divide persons into those at the earliest stage, those at a mild-to-moderate stage, and those at an advanced stage of AD, as has been done previously (Scherder, Eggermont, Visscher, Scheltens, & Swaab, 2011). Of note is that the six neuropathological stages according to Braak and Braak (1991) do not easily overlap with the three clinical stages generally used (see Textbox 1 for a clinical description of the consequences of different AD stages for daily life). Brain areas that are involved in the different play experiences are presented in Table 1.
Search Strategy
Literature searches were performed in the databases MEDLINE, Pubmed, and PsychInfo. With respect to neuropathology in persons with AD we looked for both neuropathological studies (using the following search terms: Alzheimer’s disease, neuropathology, stageing, stages, mild, moderate, severe) and neuroimaging studies (using the following search terms: Alzheimer’s disease, magnetic resonance imaging, neuroimaging, brain areas, mild, moderate, severe, temporal, parietal, frontal, occipital). With respect to studies investigating different play experiences and specific brain areas we searched for the 22 play experiences separately combining each specific experience with the search terms: magnetic resonance imaging, neuroimaging, brain areas, temporal, parietal, frontal, occipital. Finally, for the clinical studies we searched for the 22 play experiences separately in combination with the term: Alzheimer’s disease. We will first discuss our recommendations regarding the play experiences based on neuropathology and clinical studies and then our recommendations for the play experiences based on clinical studies alone.
Appropriate Play Experiences for Persons with AD Based on Neuropathology and Clinical Studies
A detailed description of our findings is presented below and a graphical presentation is given in Figure 3 in the discussion section. All brain areas that are involved in play experiences are to some degree affected in the earliest stage of AD, except for the primary somatosensory cortex (Scherder, Eggermont, Visscher, Scheltens, & Swaab, 2011; Braak & Braak, 1991; De Jong et al., 2011; Gili et al., 2011; Madsen et al., 2010; McEvoy et al., 2009; Rabinovici et al., 2007; Tondelli et al., 2012). Some areas required for play experiences, i.e., the hippocampus (Apostolova et al., 2012; Braak & Braak, 1991) and the amygdala (Roh et al., 2011; Vasconcelos et al., 2011), are affected earlier in the disease process than other areas, such as the striatum (Scherder, Eggermont, Visscher, Scheltens, & Swaab, 2011; Ginsberg et al., 2010) and anterior cingulate cortex (McDonald et al., 2009; Richards et al., 2009). For an overview of these brain areas affected by AD neuropathology see Figure 2.
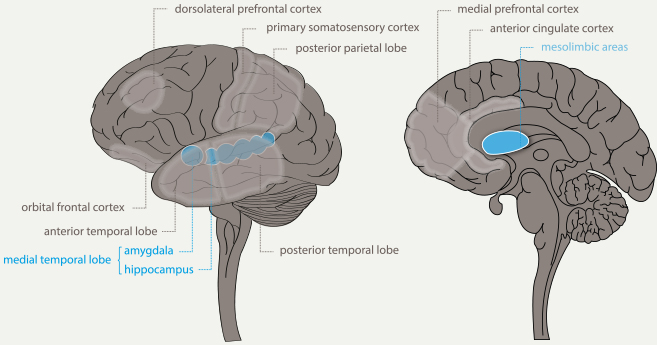
Figure 2. Relevant brain areas affected in Alzheimer’s disease. (Adapted from Lynch, 2006.)
Exploration
Active exploration of the environment is a process in which the hippocampus plays a pivotal role (Johnson et al., 2012), and this is an area affected already very early in AD (Apostolova et al., 2012). Exploration also requires executive functions (Collins & Koechlin, 2012) mediated in part by prefrontal brain areas (Declerck et al., 2006), areas that are vulnerable in AD (Rabinovici et al., 2007). Clinical studies show that the ability to take initiative is reduced in persons with AD, which is reflected in higher levels of apathy (Esposito et al., 2010; Ready, Ott, Grace, & Cahn-Weiner, 2003). We therefore recommend that game designers should not include exploratory elements appealing solely to a player’s own initiative in games for all persons with AD.
Relaxation
Sensory stimulation can lead to a state of relaxation (Poza, Gómez, Gutiérrez, Mendoza, & Hornero, 2013). Clinical studies in persons with AD generally investigate the association between sensory stimulation and measures that promote reduced agitation, rather than investigating measures that induce a feeling of relaxation. In order to reduce agitation, several sensory stimulating activities have been suggested, including massage and multisensory stimulation (Rowe & Alfred, 1999; Staal, 2012). Relaxation by sensory stimulation is therefore a recommended strategy for non-pharmacological interventions in persons with AD (Ward-Smith, Llanque, & Curran, 2009). We therefore strongly encourage inclusion of elements of sensation and relaxation in games for all people with AD, including those in an advanced stage of dementia.
Reminiscence
It is generally assumed that during reminiscence, patterns of brain activity resembling the areas that were activated during the corresponding experience are reactivated (McNaughton, 1998). Reminiscence therapy in dementia is based on the assumption that remote memory remains intact until more advanced stages of the disease (Cotelli et al., 2012). Brain areas involved in remote memory include both the posterior and anterior temporal lobes and the prefrontal lobe areas (Detour et al., 2011; Watanabe et al., 2012). In view of the widely distributed storage of elements of remote memory, people with AD appear to have relatively intact remote memory (Bayley, Gold, Hopkins, & Squire, 2005). Although remote memory deficits are present in persons with mild to moderate AD (Dorrego et al., 1999; Meeter, Eijsackers, & Mulder, 2006) remote memory indeed seems more intact than more recently stored memories, even in those with AD in an advanced stage (Sartori, Snitz, Sorcinelli, & Daum, 2004). Reminiscence as a therapy is often applied to nursing home residents with dementia (Cotelli et al., 2012). We recommend including elements of reminiscence with a personal content in the design of games for persons with AD, regardless of the stage of severity.
Sensation
The primary somatosensory cortex, the area remaining spared the longest from AD neuropathology (Braak & Braak, 1991; Jacobs et al., 2011), mediates an important play experience: sensation (Blatow, Nennig, Durst, Sartor, & Stippich, 2007; Kastrup et al., 2008). Clinical studies show that sensory systems appear to be relatively intact in persons with AD (Başar, Güntekin, Tülay, & Yener, 2010). Sensory stimulation is therefore a widely recommended strategy for non-pharmacological interventions in persons with AD (Swaab, Dubelaar, Scherder, van Someren, & Verwer, 2003; Briones, 2006).
Appropriate Play Experiences for Persons with AD Based on Clinical Studies Alone
Challenge
A task can be considered challenging when it is uncertain whether a person believes him- or herself to have sufficient ability and capacity to accomplish the task, which in turn depends on self-efficacy (Tsang, Hui, & Law, 2012). Although there are plenty of studies examining self-efficacy in caregivers of people with AD (Gallagher et al., 2011; Semiatin & O’Connor, 2012), hardly any studies focus on persons with AD themselves. As far as we know, there are no studies really describing self-efficacy measures in persons with AD, but reduced levels of self-awareness of their own memory deficits have been reported (Mimura, 2008). Elements of challenge to stimulate self-efficacy are recommended in games for persons in the earliest and mild-to-moderate stages of AD.
Eroticism
We did not find any clinical studies that examined experiences of eroticism in persons with AD, but sexual intimacy and interactions are still reported in persons with AD in a mild-to-moderate stage of the disease (Davies, Sridhar, Newkirk, Beaudreau, & O’Hara, 2012; Harris, Adams, Zubatsky, & White, 2011). Game designers can therefore consider incorporating erotic elements into games for persons in the earliest and mild-to-moderate stages of AD.
Expression
There is anecdotal evidence of clinical studies reporting expression and creativity in people with mild to moderate AD (Cummings, Miller, Christensen, & Cherry, 2008). More specifically, although persons with AD may be unable to copy images correctly or make realistic drawings, they may still be able to produce art by using colour or composition (Cummings et al., 2008). In addition, in view of communication problems often present in persons with AD (Müller & Guendouzi, 2005), a creative game may offer the possibility of expressing burdensome feelings (Przybylski, Weinstein, Murayama, Lynch, & Ryan, 2012). We recommend games that appeal to the players to express themselves for persons in the earliest and mild-to-moderate stages of AD.
Fantasy
No studies looked at the ability to fantasize in persons with AD. People with mild to moderate AD, however, are often confused about their own orientation in time and place (De Vriendt, Gorus, Bautmans, & Mets, 2012). It is therefore unclear whether the use of elements of fantasy should be recommended for persons with AD, regardless of disease stage.
Humour
There is anecdotal evidence that people with AD in a mild-to-moderate stage of the disease still have a sense of humour (Hawkins & Graff-Radford, 2007), and humour can actually help them to cope with the disease (Macrae, 2008). We therefore recommend humoristic games for persons in the earliest stage of AD, and with mild to moderate AD.
Nurturing, Fellowship, and Sympathy
Clinical studies reveal that persons with mild to moderate AD show responsiveness towards pets (Cohen-Mansfield, Marx, Thein, & Dakheel-Ali, 2010). Also, studies report friendship (Spector & Orrell, 2006) and emotional intimacy between people with mild to moderate AD (Davies et al., 2012; Harris et al., 2011). Anecdotal evidence reports that persons with mild to moderate AD can still show emotional responsiveness (Cohen-Mansfield et al., 2010). Based on these findings, we recommend games that evoke the experiences of nurture, fellowship, and sympathy for persons in the earliest and mild-to-moderate stages of AD.
Simulation
Creating a simulation of everyday life has been applied to persons with AD in the earliest and mild-to-moderate stages and was well appreciated (Hofmann et al., 2003; Pengas et al., 2012). We therefore recommend including elements of simulation for persons in the earliest and mild-to-moderate stages of AD.
Subversion
If one has knowledge concerning social norms, one might be tempted to undermine them. Persons with AD in the earliest stage have been reported to still be as capable of making decisions involving basic social norms and preferences as their age-matched counterparts (Bosch-Domènech, Nagel, & Sánchez-Andrés, 2010). We recommend games that suggest the subversion of norms for persons in the earliest stage of AD.
Suffering and Sadism
No clinical studies have been performed examining the experience of suffering or sadism in people with AD. However, due to the higher levels of anxiety (Wadsworth et al., 2012), suffering and sadism may not be suitable experiences in games for this group. Moreover, people with AD are at risk of being unable to grasp the distinction between play and real-life experience, due to their confusion about orientation in time and place (De Vriendt et al., 2012), which may cause negative or even traumatic experiences. We are therefore reluctant to recommend including elements of suffering and sadism in games for people with AD.
Thrill
No clinical studies have been performed examining thrill seeking or sensitivity to risk in people with AD. However, in general, people with AD do reveal higher levels of anxiety (Wadsworth et al., 2012). This may indirectly imply that people with AD may be less inclined to take risks. We are therefore somewhat reluctant to recommend including elements of thrill seeking in games for people with AD.
Remaining Play Experiences
No clinical studies were found examining the presence of the following experiences in persons with AD: captivation, competition, completion, control, and discovery.
Discussion
The neuropathology of AD influences a person’s capacity for and experience of playing games. To design suitable games for persons with AD, designers should know the stage of the disease and the related neuropathology, and choose the appropriate play experiences (see Figure 3). Suitable play experiences for older persons in the most advanced stage of AD are: relaxation, reminiscence, and sensation. Those with mild to moderate AD may also experience challenge, eroticism, expression, fellowship, humour, nurture, simulation, and sympathy. And those in the earliest stage of AD may also experience subversion. The play experience exploration is most likely not suitable for any older person suffering from AD. For the remaining play experiences we did not find enough evidence to draw conclusions. For design guidelines concerning play experiences for people with AD, see Textbox 2.

Figure 3. Play experiences for people with AD.
The results of this review support the design of games that specifically fit older persons in different stages of AD. An interesting existing example is the MINWii game (Benveniste, Jouvelot, & Péquignot, 2012), which is in accordance with the conclusions of our review. The MINWii game was specifically designed for—and tested with—older persons with AD and was evaluated by seven hospitalized people, all in the mild-to-moderate stage of AD (Benveniste et al., 2012). The MINWii study reported the subjects’ play behaviour while playing two different modes of the game: (1) the Improvisation Mode, based on exploration, and (2) the Challenge Mode, based on challenge and reminiscence. The disappointing findings for the Improvisation Mode are not surprising, as “exploration” relies on brain structures that are already affected in an early stage of the disease: “None of them were willing to explore the system in depth on their own, let alone improvise . . . and never even tried to click random notes” (Benveniste et al., 2012, p. 8). However, challenging the patients to copy songs of the past with the Challenge Mode was more successful: “They clearly were more comfortable following the highlighted notes than trying to create something on their own. . . . patients would now clap, sing and start reminiscing more and more often” (p. 8).
It is difficult to match the affected neuronal circuits that are responsible for eliciting play experiences to the different stages of the disease, due to the complex neuropathology of AD. However, this review represents a step in understanding the consequences of the disease for the design of games that match the cognitive abilities of people with AD. Future experimental studies, examining the play experience of people with AD in a game context, would strengthen the current theoretical knowledge from neuroimaging and neuropathology studies with more practical insights for game designers (and other designers) targeting people with dementia.
The present literature review provides game developers with evidence-based insights to design games that are suitable for persons in different stages of AD. We advise game developers to define their target group according to the level of AD because the experience of playing varies widely along the course of the disease. As we have shown in our paper, a broad variety of play experiences can potentially be successfully elicited in persons with AD. In addition, stimulating affected brain areas possibly slows down the neuropathology in that particular area according to the “use it or lose it” principle (Swaab, Dubelaar, Scherder, van Someren, & Verwer, 2003). We strongly encourage game developers to design games for persons with AD that contribute to a meaningful and fun way of spending their time. To design games that match the players’ cognitive abilities, but are also challenging and stimulating, could best be achieved through collaboration between game developers and AD specialists.
Textbox 2: Guidelines for the experience of play with Alzheimer’s Disease (AD) The severity of AD should be taken into account while designing games for people with AD, because the experience of play varies widely over the course of the disease. The play experience exploration is most likely not suitable for persons with AD, because the responsible brain areas are already affected in the earliest stages of AD. Games for people with early AD may facilitate the play experiences challenge, eroticism, expression, fellowship, humour, nurture, relaxation, reminiscence, sensation, simulation, subversion, and sympathy. Games for people with mild to moderate AD may facilitate the play experiences challenge, eroticism, expression, fellowship, humour, nurture, relaxation, reminiscence, sensation, simulation, and sympathy. Games for persons with advanced AD may facilitate the play experiences relaxation, reminiscence, and sensation. The play experience sensory stimulation is suitable for all severities of AD and therefore suitable to be incorporated into all games for people with AD. |
References
- Abler, B., Walter, H., & Erk, S. (2005). Neural correlates of frustration. Neuroreport, 16(7), 669-672.
- Apostolova, L. G., Green, A. E., Babakchanian, S., Hwang, K. S., Chou, Y. Y., Toga, A. W., & Thompson, P. M. (2012). Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment, and Alzheimer’s disease. Alzheimer Disease and Associated Disorders, 26(1), 17-27.
- Başar, E., Güntekin, B., Tülay, E., & Yener, G. G. (2010). Evoked and event related coherence of Alzheimer patients manifest differentiation of sensory–cognitive networks. Brain Research, 1357, 79-90.
- Bastos Leite, A. J., Scheltens, P., & Barkhof, F. (2004). Pathological aging of the brain: An overview. Topics in Magnetic Resonance Imaging, 15(6), 369-389.
- Baumgartner, T., Götte, L., Gügler, R., & Fehr, E. (2012). The mentalizing network orchestrates the impact of parochial altruism on social norm enforcement. Human Brain Mapping, 33(6), 1452-1469.
- Bayley, P. J., Gold, J. J., Hopkins, R. O., & Squire, L. R. (2005). The neuroanatomy of remote memory. Neuron, 46(5), 799-810.
- Benveniste, S., Jouvelot, P., & Péquignot, R. (2010). The MINWii project: Renarcissization of patients suffering from Alzheimer’s disease through video game-based music therapy. In Proceedings of the 9th Conference on Entertainment Computing (pp. 79-90). Berlin, Germany: Springer.
- Blatow, M., Nennig, E., Durst, A., Sartor, K., & Stippich, C. (2007). fMRI reflects functional connectivity of human somatosensory cortex. Neuroimage, 37(3), 927-936.
- Bosch-Domènech, A., Nagel, R., & Sánchez-Andrés, J. V. (2010). Prosocial capabilities in Alzheimer’s patients. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65(1), 119-128.
- Bouchard, B., Imbeault, F., Bouzouane, A., & Menelas, B. A. J. (2012). Developing serious games specifically adapted to people suffering from Alzheimer. In Proceedings of the 3rd Conference on Serious Games Development and Applications (pp. 243-254). Berlin, Germany: Springer.
- Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239-259.
- Briones, T. L. (2006). Environment, physical activity, and neurogenesis: Implications for prevention and treatment of Alzheimer’s disease. Current Alzheimer Research, 3(1), 49-54.
- Brunnström, H., Gustafson, L., Passant, U., & Englund, E. (2009). Prevalence of dementia subtypes: A 30-year retrospective survey of neuropathological reports. Archives of Gerontology and Geriatrics, 49(1), 146-149.
- Caillois, R. (2001). Man, play, and games. (Meyer Barash, Trans.) Champaign, IL: University of Illinois Press. (Original work published 1958)
- Cherniack, E. P. (2011). Not just fun and games: Applications of virtual reality in the identification and rehabilitation of cognitive disorders of the elderly. Disability & Rehabilitation: Assistive Technology, 6(4), 283-289.
- Cohen-Mansfield, J., Marx, M. S., Thein, K., & Dakheel-Ali, M. (2010). The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging & Mental Health, 14(1), 67-73.
- Collins, A., & Koechlin, E. (2012). Reasoning, learning, and creativity: Frontal lobe function and human decision-making. PLoS Biology, 10(3), e1001293. doi: 10.1371/journal.pbio.1001293
- Cotelli, M., Manenti, R., & Zanetti, O. (2012). Reminiscence therapy in dementia: A review. Maturitas, 72(3), 203-205.
- Cummings, J. L., Miller, B. L., Christensen, D. D., & Cherry, D. (2008). Creativity and dementia: Emerging diagnostic and treatment methods for Alzheimer’s disease. CNS Spectrums, 13(2 Suppl 2), 1-20.
- Davies, H. D., Sridhar, S. B., Newkirk, L. A., Beaudreau, S. A., & O’Hara, R. (2012). Gender differences in sexual behaviors of AD patients and their relationship to spousal caregiver well-being. Aging & Mental Health, 16(1), 89-101.
- De Jong, L. W., Ferrarini, L., van der Grond, J., Milles, J. R., Reiber, J. H., Westendorp, R., . . . van Buchem, M. A. (2011). Shape abnormalities of the striatum in Alzheimer’s disease. Journal of Alzheimer’s Disease, 23(1), 49-59.
- De Vriendt, P., Gorus, E., Bautmans, I., & Mets, T. (2012). Conversion of the mini-mental state examination to the international classification of functioning, disability and health terminology and scoring system. Gerontology, 58(2), 112-119.
- Decety, J., Jackson, P. L., Sommerville, J. A., Chaminade, T., & Meltzoff, A. N. (2004). The neural bases of cooperation and competition: An fMRI investigation. Neuroimage, 23(2), 744-751.
- Declerck, C. H., Boone, C., & De Brabander, B. (2006). On feeling in control: A biological theory for individual differences in control perception. Brain and Cognition, 62(2), 143-176.
- Detour, J., Danion, J. M., Gounot, D., Marrer, C., & Foucher, J. R. (2011). Prefrontal cortex recruitment during naturalistic remote memory: A factorial block-event fMRI study. Brain Research, 1400, 66-77.
- Dorrego, M. F., Sabe, L., Cuerva, A. G., Kuzis, G., Tiberti, C., Boller, F., & Starkstein, S. E. (1999). Remote memory in Alzheimer’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 11(4), 490-497.
- Ewers, M., Frisoni, G. B., Teipel, S. J., Grinberg, L. T., Amaro E., Jr, Heinsen, H., . . . Hampel, H. (2011). Staging Alzheimer’s disease progression with multimodality neuroimaging. Progress in Neurobiology, 95(4), 535-546.
- Esposito, F., Rochat, L., Van der Linden, A. C. J., Lekeu, F., Quittre, A., Charnallet, A., & Van der Linden, M. (2010). Apathy and executive dysfunction in Alzheimer disease. Alzheimer Disease & Associated Disorders, 24(2), 131-137.
- Falkenberg, L. E., Specht, K., & Westerhausen, R. (2011). Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain and Cognition, 76(2), 276-285.
- Farias, S. T., Park, L. Q., Harvey, D. J., Simon, C., Reed, B. R., Carmichael, O., & Mungas, D. (2013). Everyday cognition in older adults: Associations with neuropsychological performance and structural brain imaging. Journal of the International Neuropsychological Society, 19(04), 430-441.
- Flores, E., Tobon, G., Cavallaro, E., Cavallaro, F. I., Perry, J. C., & Keller, T. (2008). Improving patient motivation in game development for motor deficit rehabilitation. Proceedings of the International Conference on Advances in Computer Entertainment Technology (pp. 381-384). New York, NY: ACM.
- Franklin, R. G., Jr., & Adams, R. B., Jr. (2011). The reward of a good joke: Neural correlates of viewing dynamic displays of stand-up comedy. Cognitive, Affective, & Behavioral Neuroscience, 11(4), 508-515.
- Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology, 3(6), 343-353.
- Fua, K. C., Gupta, S., Pautler, D., & Farber, I. (2013). Designing serious games for elders. In Proceedings of the 8th International Conference on the Foundations of Digital Games (pp. 291-297). Santa Cruz, CA: SASDG.
- Gallagher, D., Ni Mhaolain, A., Crosby, L., Ryan, D., Lacey, L., Coen, R. F., . . . Lawlor, B. A. (2011). Self-efficacy for managing dementia may protect against burden and depression in Alzheimer’s caregivers. Aging & Mental Health, 15(6), 663-670.
- Gili, T., Cercignani, M., Serra, L., Perri, R., Giove, F., Maraviglia, B., . . . Bozzali, M. (2011). Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. Journal of Neurology, Neurosurgery & Psychiatry, 82(1), 58-66.
- Ginsberg, S. D., Mufson, E. J., Counts, S. E., Wuu, J., Alldred, M. J., Nixon, R. A., & Che, S. (2010). Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease, 22(2), 631-639.
- Griffiths, M. D. (2005). The therapeutic value of videogames. In J. Goldstein & J. Raessens (Eds.), Handbook of computer game studies (pp. 161-171). Boston, MA: MIT Press.
- Harenski, C. L., Thornton, D. M., Harenski, K. A., Decety, J., & Kiehl, K. A. (2012). Increased frontotemporal activation during pain observation in sexual sadism: Preliminary findings. Archives of General Psychiatry, 69(3), 283-292.
- Harris, S. M., Adams, M. S., Zubatsky, M., & White, M. (2011). A caregiver perspective of how Alzheimer’s disease and related disorders affect couple intimacy. Aging & Mental Health, 15(8), 950-960.
- Hawkins, D. B., & Graff-Radford, N. R. (2007). The ability to pun may be retained in Alzheimer disease. Neurocase, 13(1), 50-54.
- Henderson, L. A., Stathis, A., James, C., Brown, R., McDonald, S., & Macefield, V. G. (2012). Real-time imaging of cortical areas involved in the generation of increases in skin sympathetic nerve activity when viewing emotionally charged images. Neuroimage, 62(1), 30-40.
- Hofmann, M., Rösler, A., Schwarz, W., Müller-Spahn, F., Kräuchi, K., Hock, C., & Seifritz, E. (2003). Interactive computer-training as a therapeutic tool in Alzheimer’s disease. Comprehensive Psychiatry, 44(3), 213-219.
- Huizinga J. (1955). Homo ludens. A study of the play element in culture. Boston, MA: Beacon Press. (Original work published 1938)
- Ijsselsteijn, W., Nap, H. H., de Kort, Y., & Poels, K. (2007). Digital game design for elderly users. In Proceedings of the Conference on Future Play (pp. 17-22). New York, NY: ACM.
- Jacobs, H. I., Van Boxtel, M. P., Uylings, H., Gronenschild, E. H., Verhey, F. R., & Jolles, J. (2011). Atrophy of the parietal lobe in preclinical dementia. Brain and Cognition, 75(2), 154-163.
- Johnson, A., Varberg, Z., Benhardus, J., Maahs, A., & Schrater, P. (2012). The hippocampus and exploration: Dynamically evolving behavior and neural representations. Frontiers in Human Neuroscience, 6, No. 216. doi: 10.3389/fnhum.2012.00216
- Joseph, J. E., Liu, X., Jiang, Y., Lynam, D., & Kelly, T. H. (2009). Neural correlates of emotional reactivity in sensation seeking. Psychological Science, 20(2), 215-223.
- Kagerer, S., Klucken, T., Wehrum, S., Zimmermann, M., Schienle, A., Walter, B., . . . Stark, R. (2011). Neural activation toward erotic stimuli in homosexual and heterosexual males. The Journal of Sexual Medicine, 8(11), 3132-3143.
- Kastrup, A., Baudewig, J., Schnaudigel, S., Huonker, R., Becker, L., Sohns, J. M., . . . Witte, O. W. (2008). Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage, 41(4), 1364-1371.
- Kayser, A. S., Buchsbaum, B. R., Erickson, D. T., & D’Esposito, M. (2010). The functional anatomy of a perceptual decision in the human brain. Journal of Neurophysiology, 103(3), 1179-1194.
- Kern, A., & Behl, C. (2009). The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochimica et Biophysica Acta (BBA) – General Subjects, 1790(10), 1124-1132.
- Kester, M. I., & Scheltens, P. (2009). Dementia: The bare essentials. Practical Neurology, 9(4), 241-251.
- Korhonen, H., Montola, M., & Arrasvuori, J. (2009). Understanding playful user experience through digital games. In Proceedings of the International Conference on Designing Pleasurable Products and Interfaces (pp. 274-285). New York, NY: ACM.
- Kühn, S., & Gallinat, J. (2011). A quantitative meta-analysis on cue-induced male sexual arousal. The Journal of Sexual Medicine, 8(8), 2269-2275.
- Lewis, G. N., & Rosie, J. A. (2012). Virtual reality games for movement rehabilitation in neurological conditions: How do we meet the needs and expectations of the users? Disability and Rehabilitation, 34(22), 1880-1886.
- Lucero, A., & Arrasvuori, J. (2010). PLEX Cards: A source of inspiration when designing for playfulness. In Proceedings of the 3rd International Conference on Fun and Games (pp. 28-37). New York, NY: ACM.
- Lucero, M., Kijek, J., Malone, L., Santos, R., & Hendrix, K. (2000). Products for Alzheimer’s patients with “null” behavior. American Journal of Alzheimer’s Disease and Other Dementias, 15(6), 347-356.
- Lynch, P. (2006). Brain human lateral view. Retrieved April 25, 2015, from https://commons.wikimedia.org/wiki/File:Brain_human_lateral_view.svg
- Macrae, H. (2008). ‘Making the best you can of it’: Living with early-stage Alzheimer’s disease. Sociology of Health & Illness, 30(3), 396-412.
- Madsen, S. K., Ho, A. J., Hua, X., Saharan, P. S., Toga, A. W., Jack, C. R., Jr, . . . Thompson, P. M. (2010). 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiology of Aging, 31(8), 1312-1325.
- Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., & Macrae, C. N. (2007). Wandering minds: The default network and stimulus-independent thought. Science, 315(5810), 393-395.
- McCallum, S., & Boletsis, C. (2013). Dementia games: A literature review of dementia-related serious games. In Proceedings of the 4th Conference on Serious Games Development and Applications (pp. 15-27). Berlin, Germany: Springer.
- McDonald, C. R., McEvoy, L. K., Gharapetian, L., Fennema-Notestine, C., Hagler, D. J., Holland, D., . . . Dale, A. M. (2009). Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology, 73(6), 457-465.
- McEvoy, L. K., Fennema-Notestine, C., Roddey, J. C., Hagler D. J., Jr, Holland, D., Karow, D. S., . . . Dale, A. M. (2009). Alzheimer disease: Quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology, 251(1), 195-205.
- McNaughton, B. L. (1998). The neurophysiology of reminiscence. Neurobiology of Learning and Memory, 70(1), 252-267.
- Meeter, M., Eijsackers, E. V., & Mulder, J. L. (2006). Retrograde amnesia for autobiographical memories and public events in mild and moderate Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 28(6), 914-927.
- Mimura, M. (2008). Memory impairment and awareness of memory deficits in early-stage Alzheimer’s disease. Tohoku Journal of Experimental Medicine, 215(2), 133-140.
- Mobbs, D., Greicius, M. D., Abdel-Azim, E., Menon, V., & Reiss, A. L. (2003). Humor modulates the mesolimbic reward centers. Neuron, 40(5), 1041-1048.
- Mohr, P. N., Biele, G., & Heekeren, H. R. (2010). Neural processing of risk. The Journal of Neuroscience, 30(19), 6613-6619.
- Morey, R. A., McCarthy, G., Selgrade, E. S., Seth, S., Nasser, J. D., & LaBar, K. S. (2012). Neural systems for guilt from actions affecting self versus others. Neuroimage, 60(1), 683-692.
- Morris, J. C. (1993). The clinical dementia rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412-2414.
- Müller, N., & Guendouzi, J. A. (2005). Order and disorder in conversation: Encounters with dementia of the Alzheimer’s type. Clinical Linguistics & Phonetics, 19(5), 393-404.
- Nelson, P. T., Head, E., Schmitt, F. A., Davis, P. R., Neltner, J. H., Jicha, G. A., . . . Scheff, S. W. (2011). Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathologica, 121(5), 571-587.
- Palmiero, M., Di Giacomo, D., & Passafiume, D. (2012). Creativity and dementia: A review. Cognitive Processing, 13(3), 193-209.
- Pengas, G., Williams, G. B., Acosta-Cabronero, J., Ash, T. W., Hong, Y. T., Izquierdo-Garcia, D., . . . Nestor, P. J. (2012). The relationship of topographical memory performance to regional neurodegeneration in Alzheimer’s disease. Front Aging Neuroscience, 4, No. 17. doi: 10.3389/fnagi.2012.00017.
- Poza, J., Gómez, C., Gutiérrez, M. T., Mendoza, N., & Hornero, R. (2013). Effects of a multi-sensory environment on brain-injured patients: Assessment of spectral patterns. Medical Engineering & Physics, 35(3), 365-375.
- Przybylski, A. K., Weinstein, N., Murayama, K., Lynch, M. F., & Ryan, R. M. (2012). The ideal self at play: The appeal of video games that let you be all you can be. Psychological Science, 23(1), 69-76.
- Qiu, C., de Ronchi, D., & Fratiglioni, L. (2007). The epidemiology of the dementias: An update. Current Opinion in Psychiatry, 20(4), 380-385.
- Rabinovici, G. D., Seeley, W. W., Kim, E. J., Gorno-Tempini, M. L., Rascovsky, K., Pagliaro, T. A., . . . Rosen, H. J. (2007). Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. American Journal of Alzheimer’s Disease and Other Dementias, 22(6), 474-488.
- Ready, R. E., Ott, B. R., Grace, J., & Cahn-Weiner, D. A. (2003). Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. The American Journal of Geriatric Psychiatry, 11(2), 222-228.
- Reisberg, B., Ferris, S. H., de Leon, M. J., & Crook, T. (1982). The global deterioration scale for assessment of primary degenerative dementia. The American Journal of Psychiatry, 139(9), 1136-1139.
- Reuter, M., Weber, B., Fiebach, C. J., Elger, C., & Montag, C. (2009). The biological basis of anger: Associations with the gene coding for DARPP-32 (PPP1R1B) and with amygdala volume. Behavioural Brain Research, 202(2), 179-183.
- Richards, B. A., Chertkow, H., Singh, V., Robillard, A., Massoud, F., Evans, A. C., & Kabani, N. J. (2009). Patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Neurobiology of Aging, 30(10), 1626-1636.
- Rilling, J. K. (2013). The neural and hormonal bases of human parental care. Neuropsychologia, 51(4), 731-747.
- Robert, P. H., König, A., Amieva, H., Andrieu, S., Bremond, F., Bullock, R., . . . Manera, V. (2014). Recommendations for the use of serious games in people with Alzheimer’s disease, related disorders and frailty. Frontiers in Aging Neuroscience, 6, No. 54.
- Roelofs, K., Minelli, A., Mars, R. B., van Peer, J., & Toni, I. (2009). On the neural control of social emotional behavior. Social Cognitive and Affective Neuroscience, 4(1), 50-58.
- Roh, J. H., Park, M. H., Ko, D., Park, K. W., Lee, D. H., Han, C., . . . Jung, K. Y. (2011). Region and frequency specific changes of spectral power in Alzheimer’s disease and mild cognitive impairment. Clinical Neurophysiology, 122(11), 2169-2176.
- Rosenberg, D. R., Dick, E. L., O’Hearn, K. M., & Sweeney, J. A. (1997). Response-inhibition deficits in obsessive-compulsive disorder: An indicator of dysfunction in frontostriatal circuits. Journal of Psychiatry and Neuroscience, 22(1), 29-38.
- Rowe, M., & Alfred, D. (1999). The effectiveness of slow-stroke massage in diffusing agitated behaviors in individuals with Alzheimer’s disease. Journal of Gerontological Nursing, 25(6), 22-34.
- Samanta, M. K., Wilson, B., Santhi, K., Kumar, K. S., & Suresh, B. (2006). Alzheimer disease and its management: A review. American Journal of Therapeutics, 13(6), 516-526.
- Sartori, G., Snitz, B. E., Sorcinelli, L., & Daum, I. (2004). Remote memory in advanced Alzheimer’s disease. Archives of Clinical Neuropsychology, 19(6), 779-789.
- Scherder, E. J., Bogen, T., Eggermont, L. H., Hamers, J. P., Swaab, D. F. (2010). The more physical inactivity, the more agitation in dementia. International Psychogeriatrics, 22(8), 1203-8.
- Scherder, E. J., Eggermont, L. H., Visscher, C, Scheltens, P., Swaab, D. (2011). Understanding higher level gait disturbances in mild dementia in order to improve rehabilitation: ‘Last in-first out.’ Neuroscience & Biobehavioral Reviews 35(3), 699-714.
- Semiatin, A. M., & O’Connor, M. K. (2012). The relationship between self-efficacy and positive aspects of caregiving in Alzheimer’s disease caregivers. Aging & Mental Health, 16(6), 683-688.
- Sharkey, N., & Sharkey, A. (2011). The eldercare factory. Gerontology, 58(3), 282-288.
- Spector, A., & Orrell, M. (2006). Quality of life (QoL) in dementia: A comparison of the perceptions of people with dementia and care staff in residential homes. Alzheimer Disease & Associated Disorders, 20(3), 160-165.
- Staal, J. A. (2012). Functional analytic multisensory environmental therapy for people with dementia. International Journal of Alzheimer’s Disease, 2012, No. 294801. http://dx.doi.org/10.1155/2012/294801
- Swaab, D. F., Dubelaar, E. J., Scherder, E. J., van Someren, E. J., Verwer, R. W. (2003). Therapeutic strategies for Alzheimer disease: Focus on neuronal reactivation of metabolically impaired neurons. Alzheimer Disease & Associated Disorders, 17 Suppl 4: S114-22.
- Szpunar, K. K., Jacques, P. L. S., Robbins, C. A., Wig, G. S., & Schacter, D. L. (2013). Repetition-related reductions in neural activity reveal component processes of mental simulation. Social Cognitive and Affective Neuroscience, 9(5), 712-722.
- Van Rijn, H., van Hoof, J., & Stappers, P. J. (2010). Designing leisure products for people with dementia: Developing “the chitchatters” game. American Journal of Alzheimer’s Disease and Other Dementias, 25(1), 74-89.
- Tondelli, M., Wilcock, G. K., Nichelli, P., De Jager, C. A., Jenkinson, M., & Zamboni, G. (2012). Structural MRI changes detectable up to ten years before clinical Alzheimer’s disease. Neurobiology of Aging, 33(4), 825.e25-825.e36.
- Tsang, S. K., Hui, E. K., & Law, B. C. (2012). Self-efficacy as a positive youth development construct: A conceptual review. The Scientific World Journal, 2012, Article 452327. http://dx.doi.org/10.1100/2012/452327
- Vasconcelos, L. D. G., Jackowski, A. P., Oliveira, M. O. D., Flor, Y. M. R., Bueno, O. F. A., & Brucki, S. M. D. (2011). Voxel-based morphometry findings in Alzheimer’s disease: Neuropsychiatric symptoms and disability correlations – Preliminary results. Clinics, 66(6), 1045-1050.
- Villemagne, V. L., & Rowe, C. C. (2013). Long night’s journey into the day: Amyloid-β imaging in Alzheimer’s disease. Journal of Alzheimer’s Disease, 33, S349-S359.
- Viskontas, I. V., Possin, K. L., & Miller, B. L. (2007). Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Annals of the New York Academy of Sciences, 1121(1), 528-545.
- Wadsworth, L. P., Lorius, N., Donovan, N. J., Locascio, J. J., Rentz, D. M., Johnson, K., . . . Marshall, G. A. (2012). Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dementia and Geriatric Cognitive Disorders, 34(2), 96-111.
- Watanabe, T., Kimura, H. M., Hirose, S., Wada, H., Imai, Y., Machida, T., . . . & Konishi, S. (2012). Functional dissociation between anterior and posterior temporal cortical regions during retrieval of remote memory. The Journal of Neuroscience, 32(28), 9659-9670.
- Ward-Smith, P., Llanque, S. M., & Curran, D. (2009). The effect of multisensory stimulation on persons residing in an extended care facility. American Journal of Alzheimer’s Disease and Other Dementias, 24(6), 450-455.
- Whitcomb, G. R. (1990). Computer games for the elderly. ACM SIGCAS Computers and Society, 20(3), 112-115.
- Zaki, J., Schirmer, J., & Mitchell, J. P. (2011). Social influence modulates the neural computation of value. Psychological Science, 22(7), 894-900.

